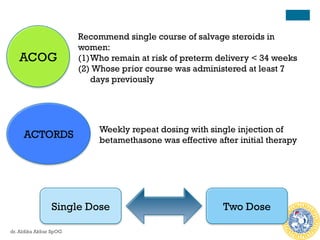
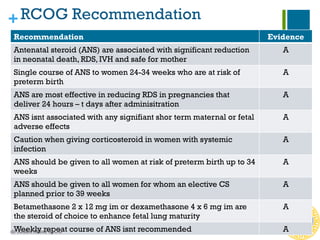
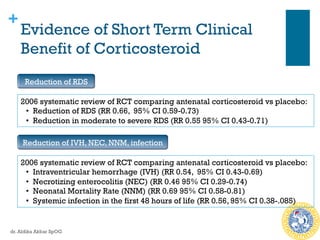
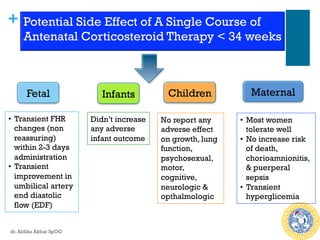
Antenatal corticosteroids are effective in reducing risks for preterm infants when administered to mothers at risk of preterm birth between 24-34 weeks of gestation. A single course of corticosteroids, such as betamethasone or dexamethasone, significantly reduces the risks of respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis and improves neonatal outcomes with no significant maternal risks. While a single repeat or rescue course may provide additional benefits, multiple courses are not recommended due to potential risks of impaired fetal growth and cerebral palsy.


![+
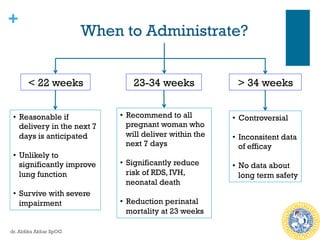
Corticosteroid Use At 37-39 Weeks
n Empiric use of steroid has been recommended prior to CS at 37-39
weeks
n The ASTECS trial, 1000 women, betamethasone vs placebo, 48 hours
before planned CS:
n Reduction in overall incidence of repiratory problems (Transient
tachypnea of the newborn and RDS) [RR 0.46]
n Similar trial, dexamethasone vs placebo, 48 hours before, 38 weeks
planned CS:
n Reduction in NICU admission for repiratory morbidity
n Reduction in transient tachypnea of the newborn (RR 0.38)
n No difference in RDS, need for mechanical ventilation
dr. Aldika Akbar SpOG](https://image.slidesharecdn.com/cmesteroidtokolitikpreterm-160515112321/85/Antenatal-Steroid-for-Preterm-Birth-8-320.jpg)



![+
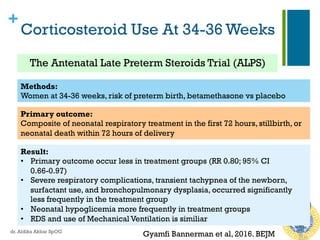
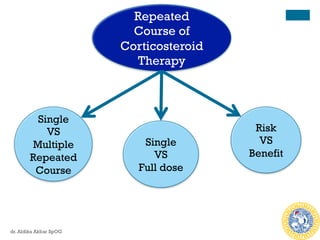
Efficacy of Repeated Course of
Steroid Therapy
n 2015 systematic review RCT, repeated doses
betamethasone vs placebo
n RESULT
n For the neonate:
n Reduced risk of RDS (RR: 0.83)
n Reduced risk composite serious infant outcome [RR 0.84]
(perinatal death, bronchopulmonary dysplasia, IVH, NEC,
sepsis, periventricular leucomalacia, & ROP)
n For the mothers: no difference in chorioamnionitis & puerpural
sepsis
n For the young child: no significant benefit & harm
dr. Aldika Akbar SpOG](https://image.slidesharecdn.com/cmesteroidtokolitikpreterm-160515112321/85/Antenatal-Steroid-for-Preterm-Birth-17-320.jpg)