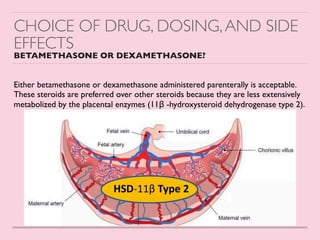
This document discusses the use of antenatal corticosteroids (ACS) to improve outcomes for preterm infants. It finds that ACS reduces rates of respiratory distress syndrome, mortality, intraventricular hemorrhage and necrotizing enterocolitis in preterm neonates when administered to mothers between 24-34 weeks of gestation who are at risk of preterm birth within 7 days. The mechanisms of action include accelerating lung development. Either betamethasone or dexamethasone can be used, though dexamethasone is preferred in India due to drug availability. Guidelines recommend ACS use in specified high-risk populations, but not as routine care before elective c-sections or beyond 34 weeks