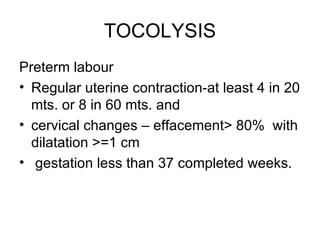
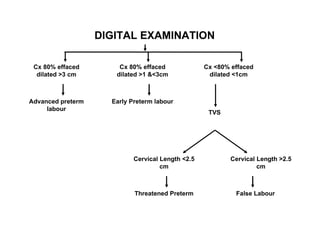
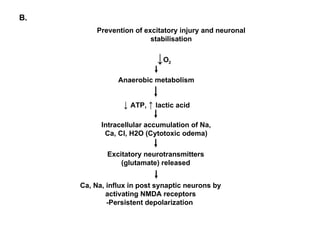
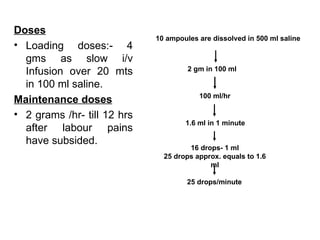
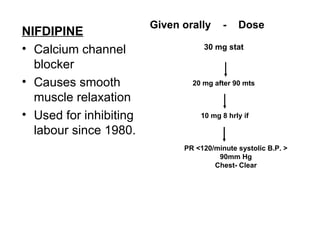
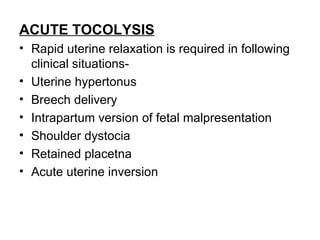
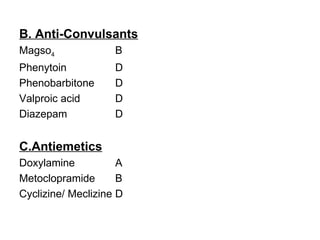
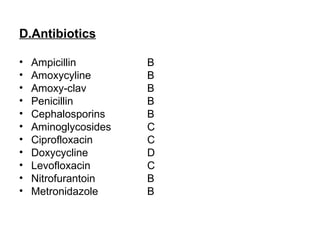
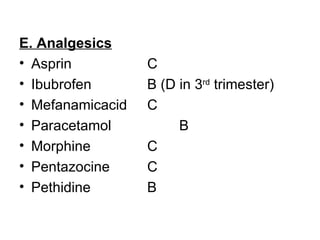
1. The document discusses various tocolytic agents used to inhibit uterine contractions in preterm labor, including magnesium sulfate, beta-adrenergic agonists like terbutaline, calcium channel blockers like nifedipine, prostaglandin synthetase inhibitors like indomethacin, nitroglycerin, diazoxide, atosiban, and ethyl alcohol.
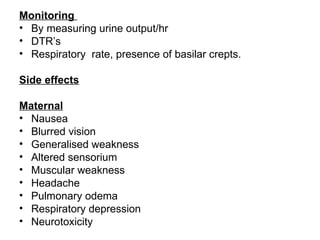
2. Each drug is described in terms of its mechanism of action in relaxing the uterus, recommended dosages, potential side effects on mother and fetus, and contraindications. Monitoring is important when using tocolytic drugs to prevent side effects like hypotension, respiratory depression,