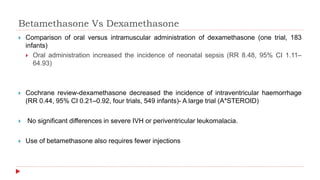
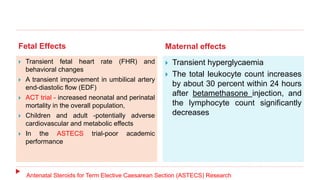
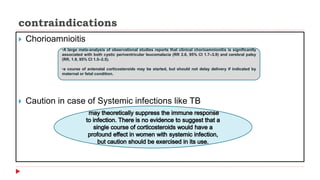
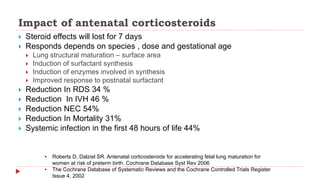
This document discusses the use of antenatal steroids to accelerate fetal lung maturation and reduce complications of prematurity. It finds that a single course of antenatal corticosteroids between 24-34 weeks gestation significantly reduces rates of respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, and mortality in preterm infants. While betamethasone and dexamethasone are commonly used, betamethasone is preferred due to fewer required doses. The benefits last up to 7 days, so treatment should be considered if delivery is anticipated within the next week.



![ 34 to 36 weeks of gestation- The Antenatal Late Preterm Steroids Trial
(ALPS)
Composite of neonatal respiratory treatment in the first 72 hours (11.6 versus 14.4
percent; relative risk [RR] 0.80; 95% CI 0.66-0.97
Severe respiratory complications (CPAP or high flow nasal cannula for ≥12 hours,
FIO2≥0.30 for at least 24 hours), transient tachypnea of the newborn, surfactant use, and
bronchopulmonary dysplasia.
Neonatal hypoglycemia
Rates of respiratory distress syndrome (RDS) and mechanical ventilation- no difference
ACOG , RCOG
37 to 39 weeks of gestation -The Antenatal Steroids for Term Caesarean
Section trial (ASTECS)
reduction in the overall incidence of respiratory problems in the treated group](https://image.slidesharecdn.com/antenatalsteroids-180923150505/85/Antenatal-steroids-6-320.jpg)