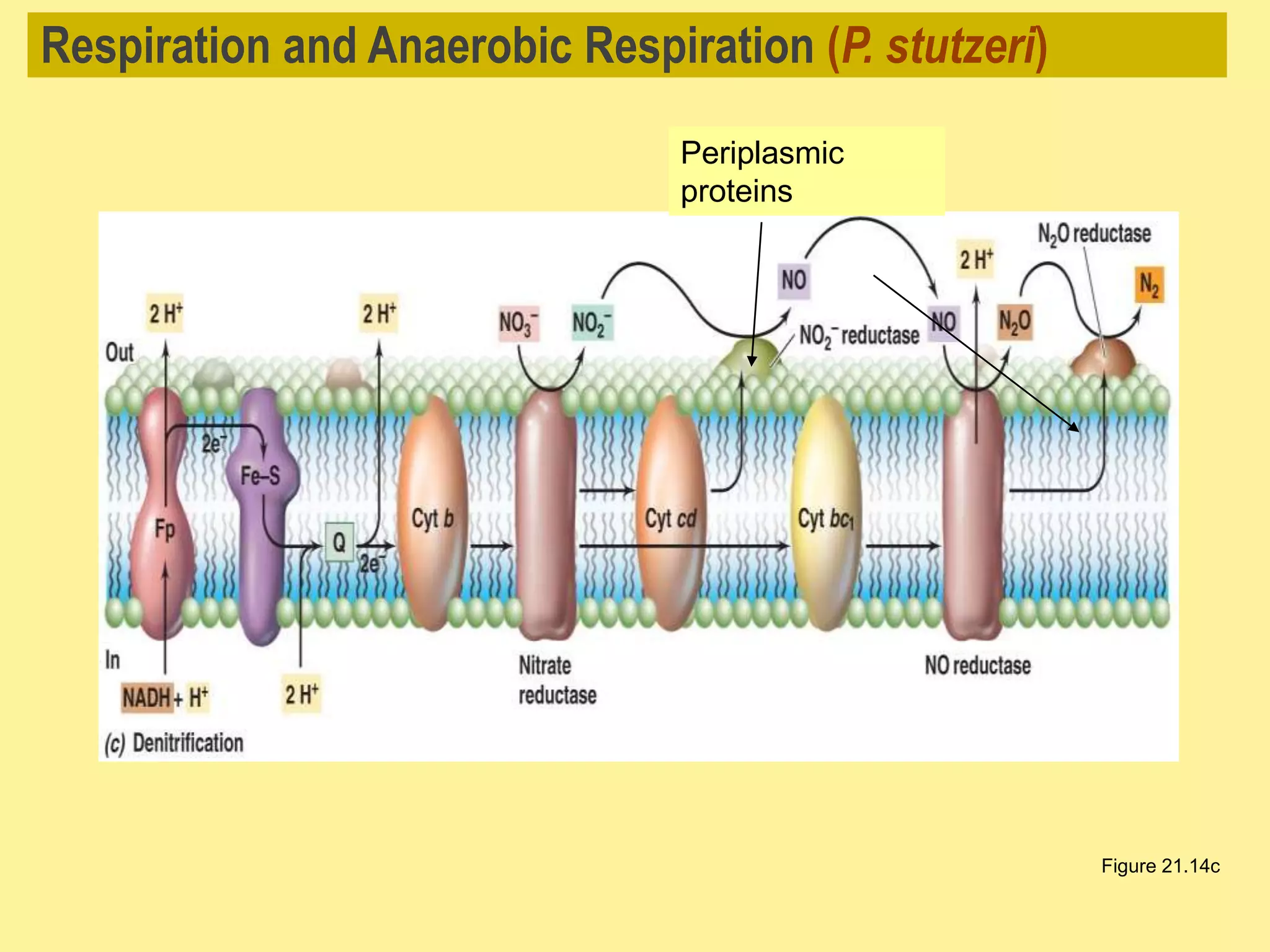
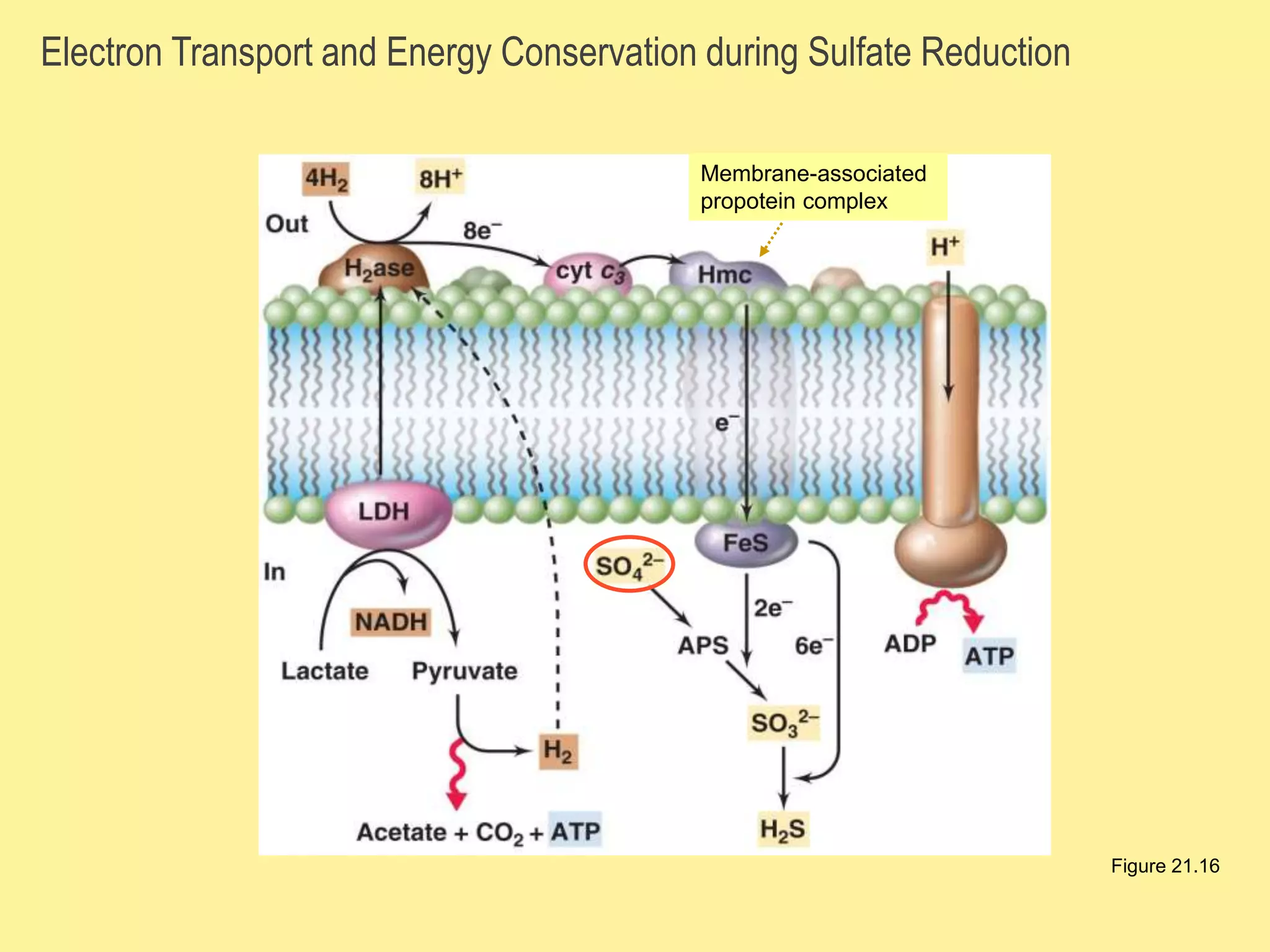
This document discusses different types of anaerobic respiration. It explains that anaerobic respiration uses inorganic molecules other than oxygen as the final electron acceptor, such as sulfur, nitrate, carbon dioxide or metals. It provides examples of denitrification and sulfate reduction, two types of anaerobic respiration where nitrate and sulfate are used as electron acceptors. The document also notes that anaerobic respiration produces less energy than aerobic respiration due to fewer protons being pumped out during electron transport.