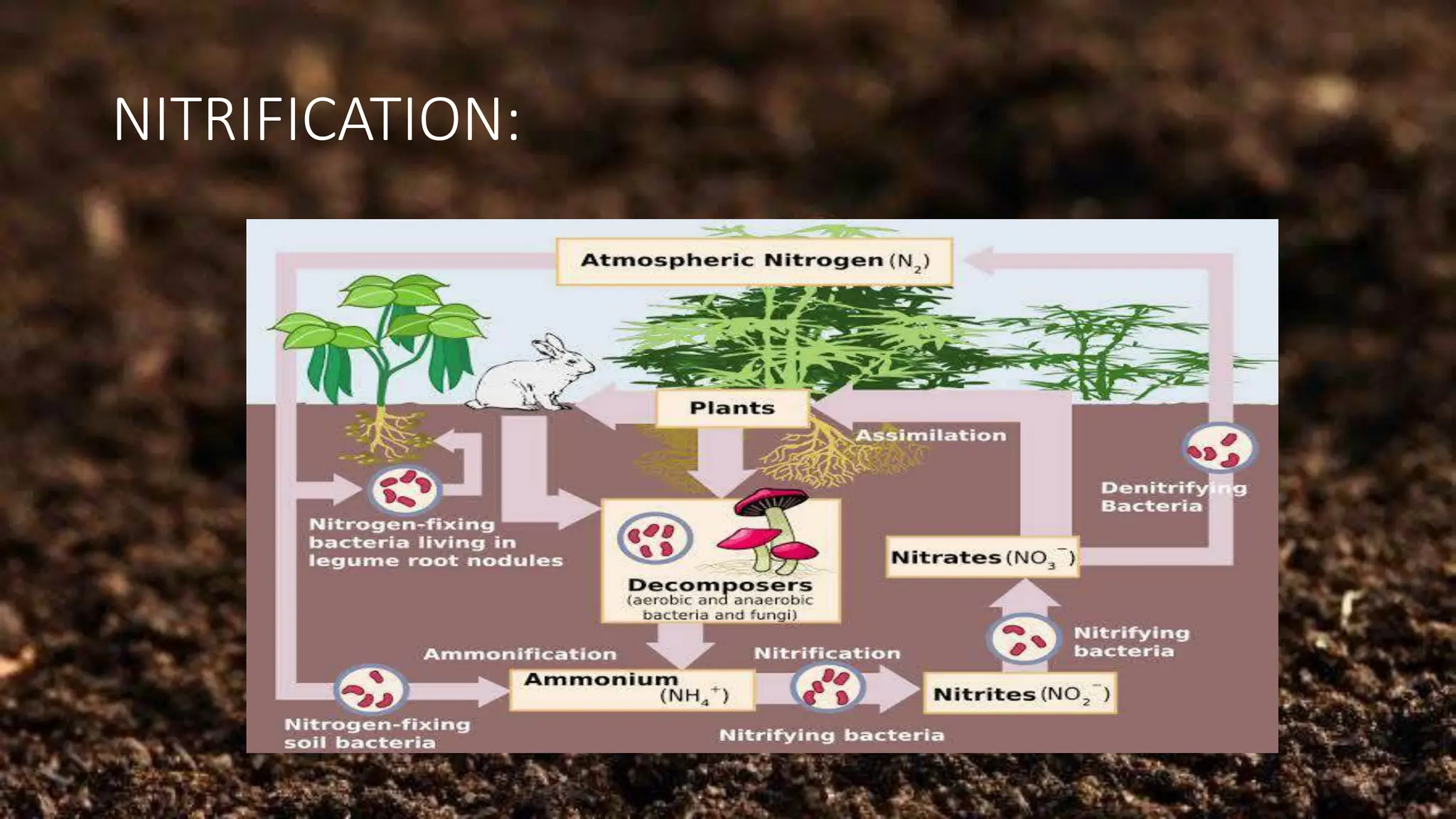
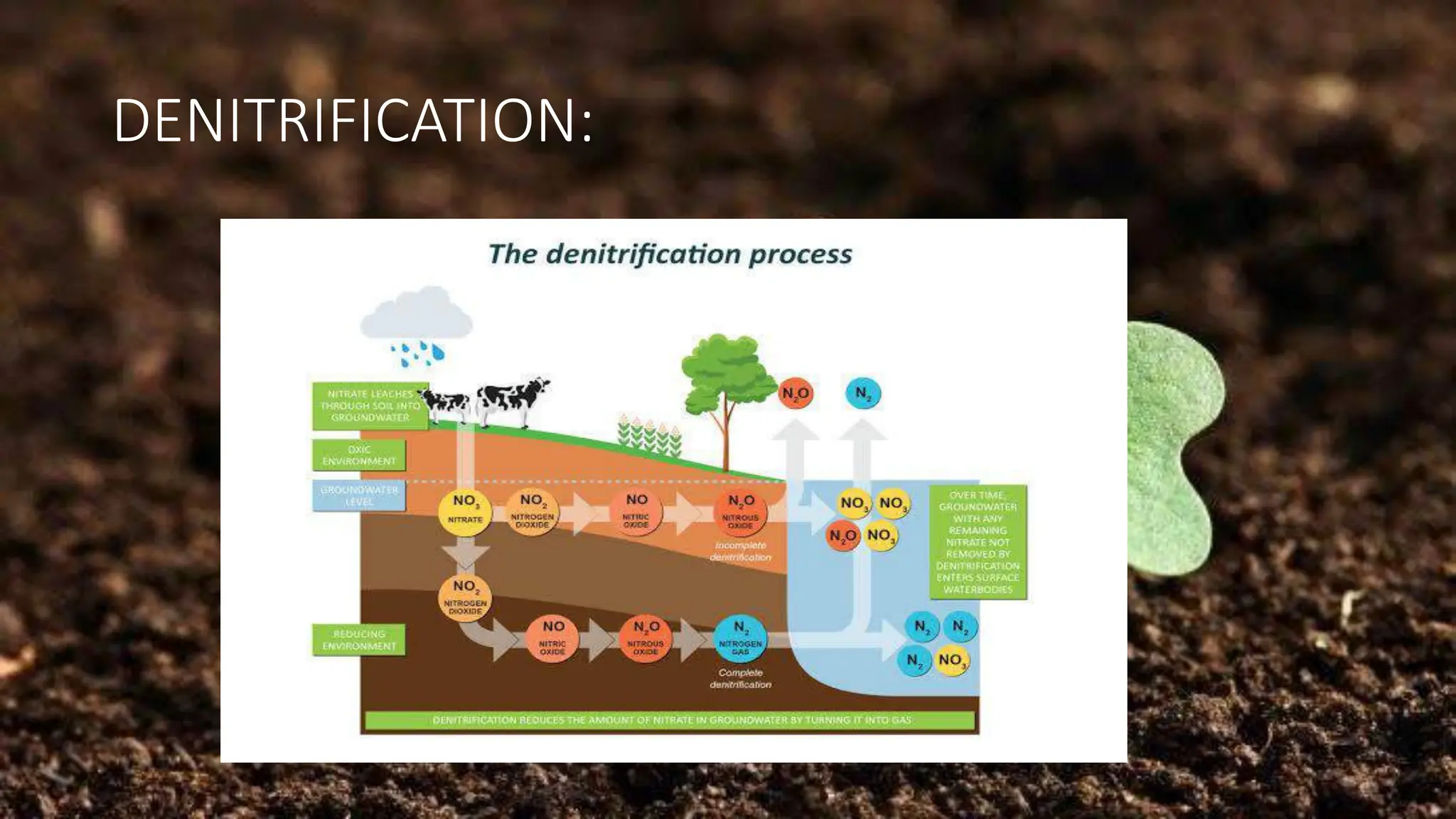
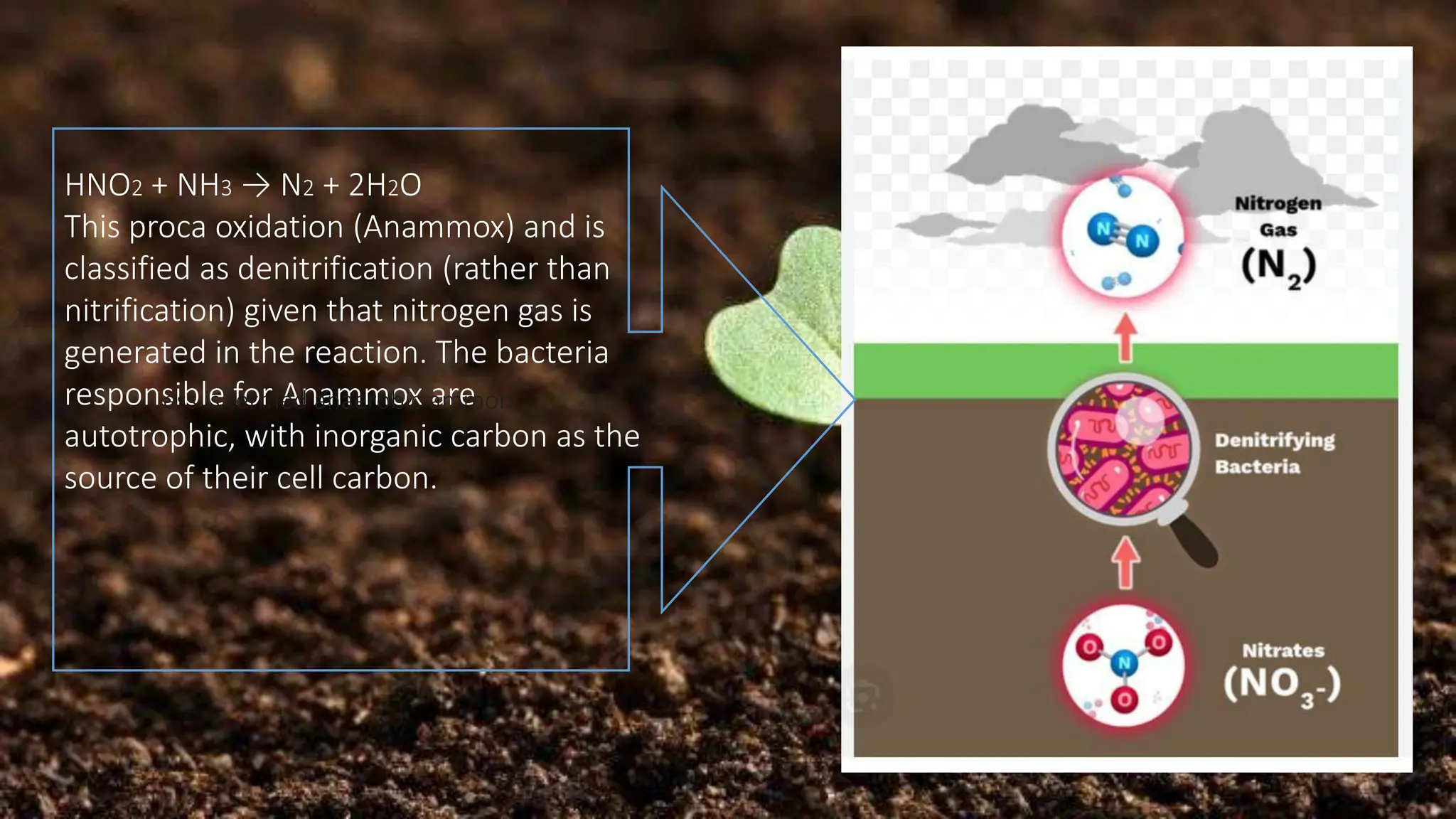
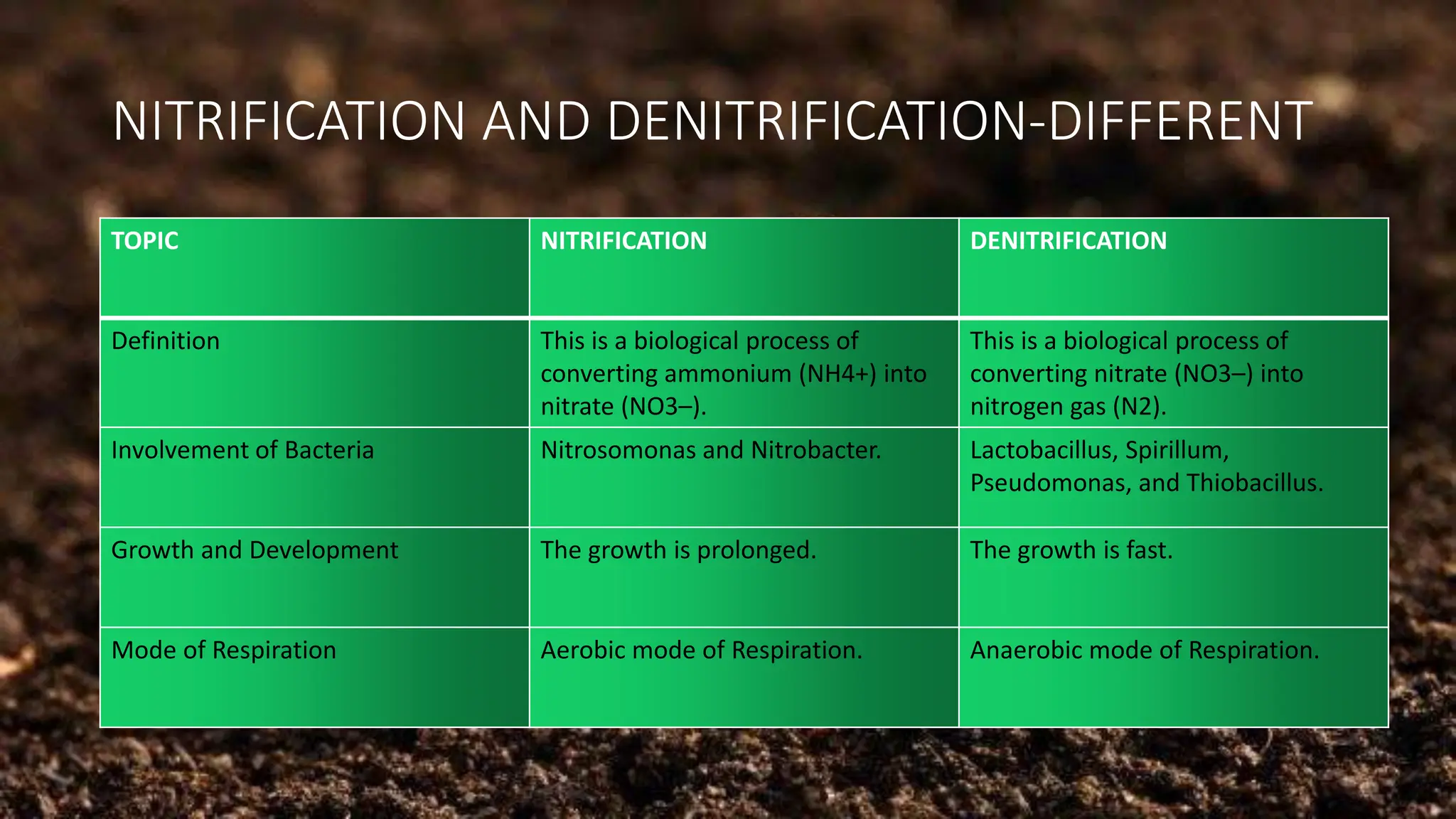
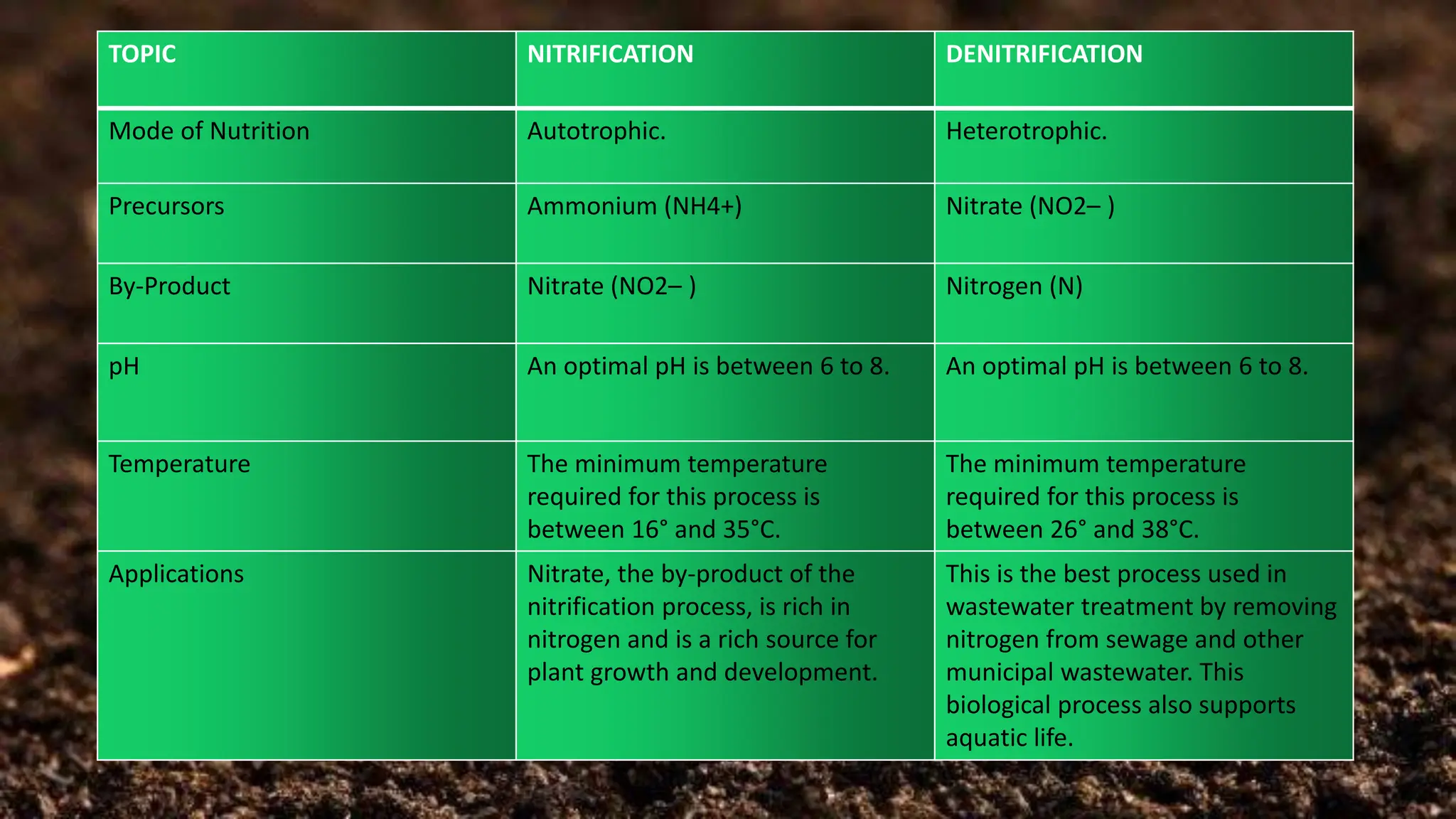
The document discusses the processes of nitrification and denitrification in soil, detailing the specific bacteria involved in each process. Nitrification converts ammonia into nitrate through bacteria such as Nitrosomonas and Nitrobacter, while denitrification reduces nitrate into nitrogen gas using bacteria like Pseudomonas and Clostridium. It highlights the importance of these processes in maintaining soil health and preventing loss of soil fertility.