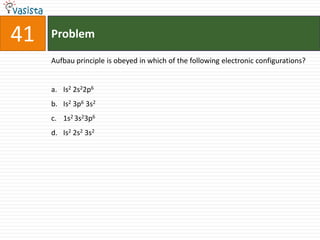
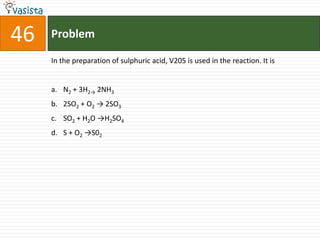
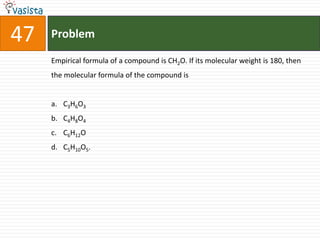
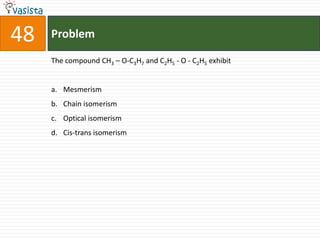
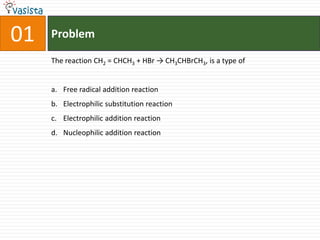
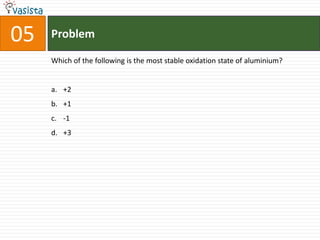
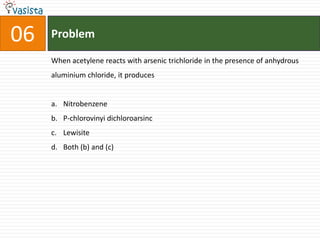
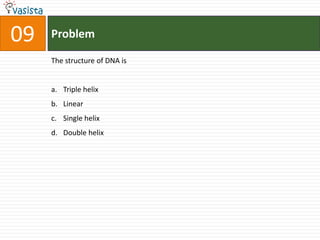
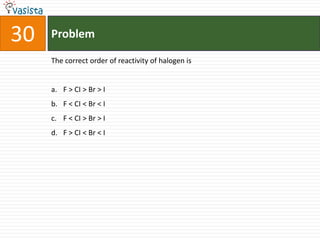
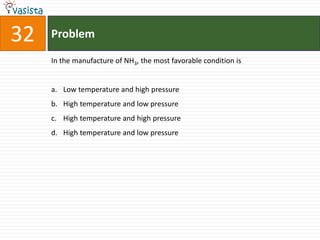
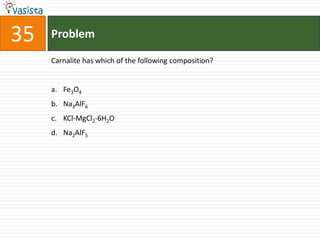
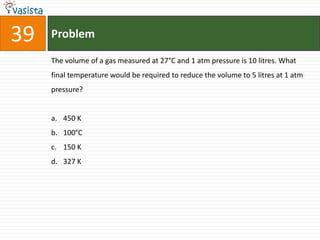
This document contains a 50 question chemistry exam with multiple choice answers for each question. The exam covers topics such as reaction types, chemical properties, molecular structures, oxidation states, and more. Each question provides 4 possible answer choices with only one being correct. Candidates are asked to indicate their choice of the correct answer in their answer book.
















![Problem21Nessler's reagent is represented byK2[HgI4] HgOHgI2K2[HgI2]Hgl2](https://image.slidesharecdn.com/chemistry-1999-111007062205-phpapp02/85/AFMC-Chemistry-1999-23-320.jpg)












![Problem40The number of electrons in [19K40]-1 is181920 40](https://image.slidesharecdn.com/chemistry-1999-111007062205-phpapp02/85/AFMC-Chemistry-1999-42-320.jpg)