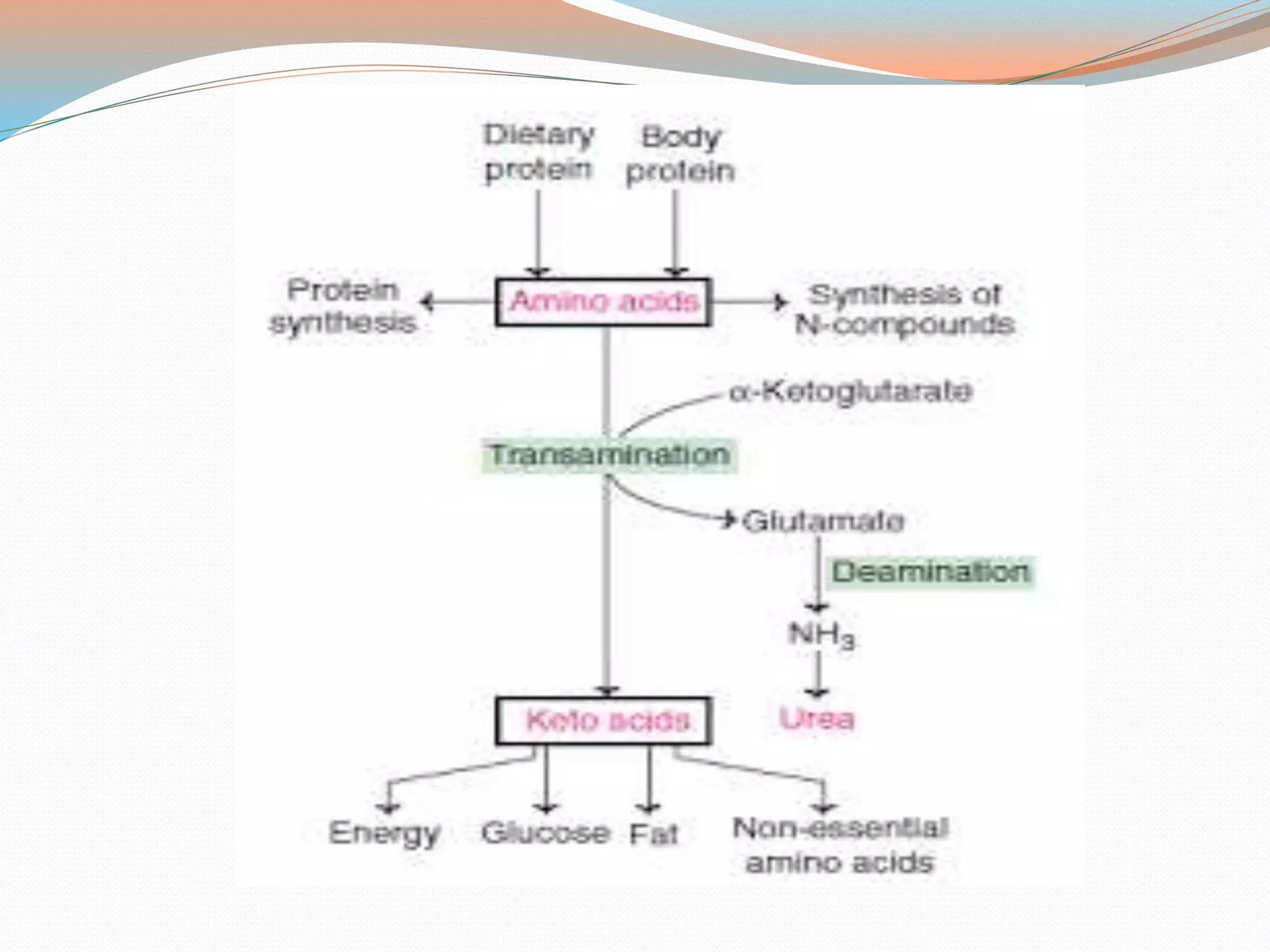
The document provides a comprehensive overview of protein metabolism and the associated biochemical processes, highlighting the significance of amino acids and their pool in the body. It explains transamination and deamination, detailing the roles of enzymes, coenzymes, and the urea cycle, which is crucial for detoxifying ammonia by converting it to urea. Additionally, it outlines the steps involved in the urea cycle, emphasizing its energy requirements and the fate of urea in the body.