Embed presentation
Downloaded 11 times

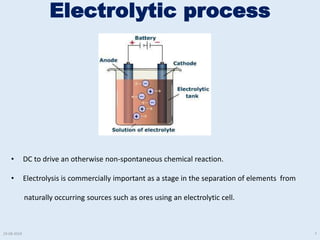




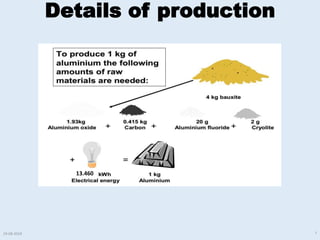




The document discusses electrolytic processes and smelting used in aluminum production. Bauxite ore is first mined and processed through crushing, milling, and the Bayer process to produce alumina. The alumina is then smelted using the Hall-Heroult process where aluminum ions are reduced at the cathode using an electrolytic cell with carbon anodes and molten cryolite as the electrolyte. Heat over 900°C is required for the electrolytic reduction to produce liquid aluminum and oxygen gas. Images and videos show various stages of the aluminum production process.










