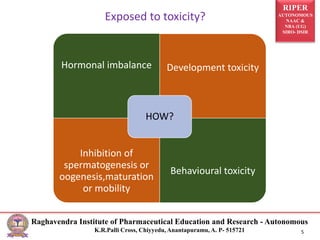
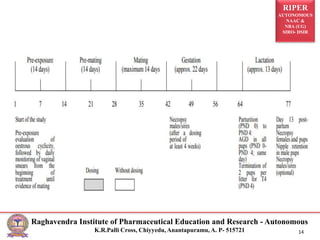
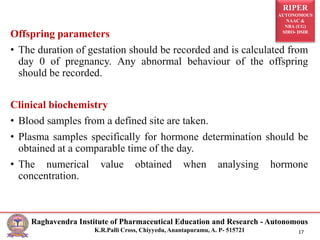
This document outlines the guidelines and methodologies for conducting reproductive toxicity studies on male fertility. It discusses various aspects such as definitions, test administration procedures, experimental schedules, and data reporting, following OECD recommendations. Data collected will evaluate toxic effects on both male and female reproductive systems, focusing on structural and functional changes impacting fertility.