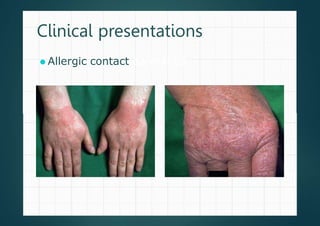
Allergic contact dermatitis (ACD) is a common inflammatory skin disease resulting from direct or indirect contact with allergens, primarily affecting those with prior sensitization. The prevalence varies significantly between populations, with diagnoses often confirmed through patch testing and careful clinical evaluation. Treatment involves allergen avoidance, topical corticosteroids, and potentially systemic corticosteroids for severe cases.









![Investigation : Patch testing 1
⚫ Indicated in patients with chronic, pruritic eczematous or
lichenified dermatitis in whom ACD is suspected
⚫ Affected by
⚫ Oral corticosteroid [>20 mg of prednisolone/day or
equivalent]
⚫ Cancer chemotherapy or immunosuppressive drug
⚫ Topical corticosteroid should be discontinued for 5-7 days
before patch testing
⚫ Not affected by antihistamines](https://image.slidesharecdn.com/shikharacdderma-240228123931-b7da2885/85/Allergic-contact-dermatitis-shikhar-pptx-11-320.jpg)































