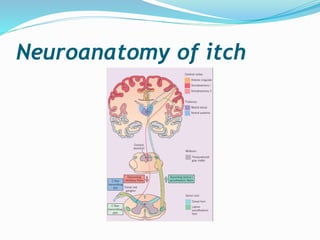
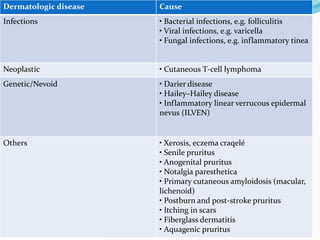
This document discusses the definition, measurement, pathophysiology, receptors, mediators, and causes of pruritus (itch). It defines pruritus as a subjective unpleasant sensation that elicits an immediate desire to scratch. Measurement methods in animals and humans are described. The pathophysiology involves polymodal nociceptors and transient receptor potential ion channels. Mediators like histamine, proteinases, substance P, opioids, neurotrophins, prostanoids, cytokines and acetylcholine are discussed. Causes include systemic disease, dermatological conditions like atopic dermatitis, infections, neoplasms, genetic disorders and others.







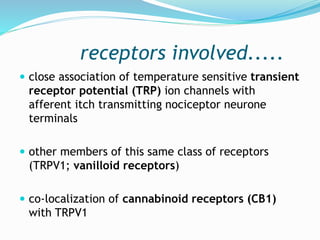
![receptors involved.....
Apart from TRPV1 ion channels,
keratinocytes also express voltage-gated
ATP channels, adenosine receptors,
cannabinoid receptors, opioid receptors
and PAR-2 (proteinase activated
receptors-2) neurotrophic tyrosine
kinase receptor type 1 (NTRK1[TRKA])](https://image.slidesharecdn.com/pruritus-180307062543/85/Pruritus-9-320.jpg)