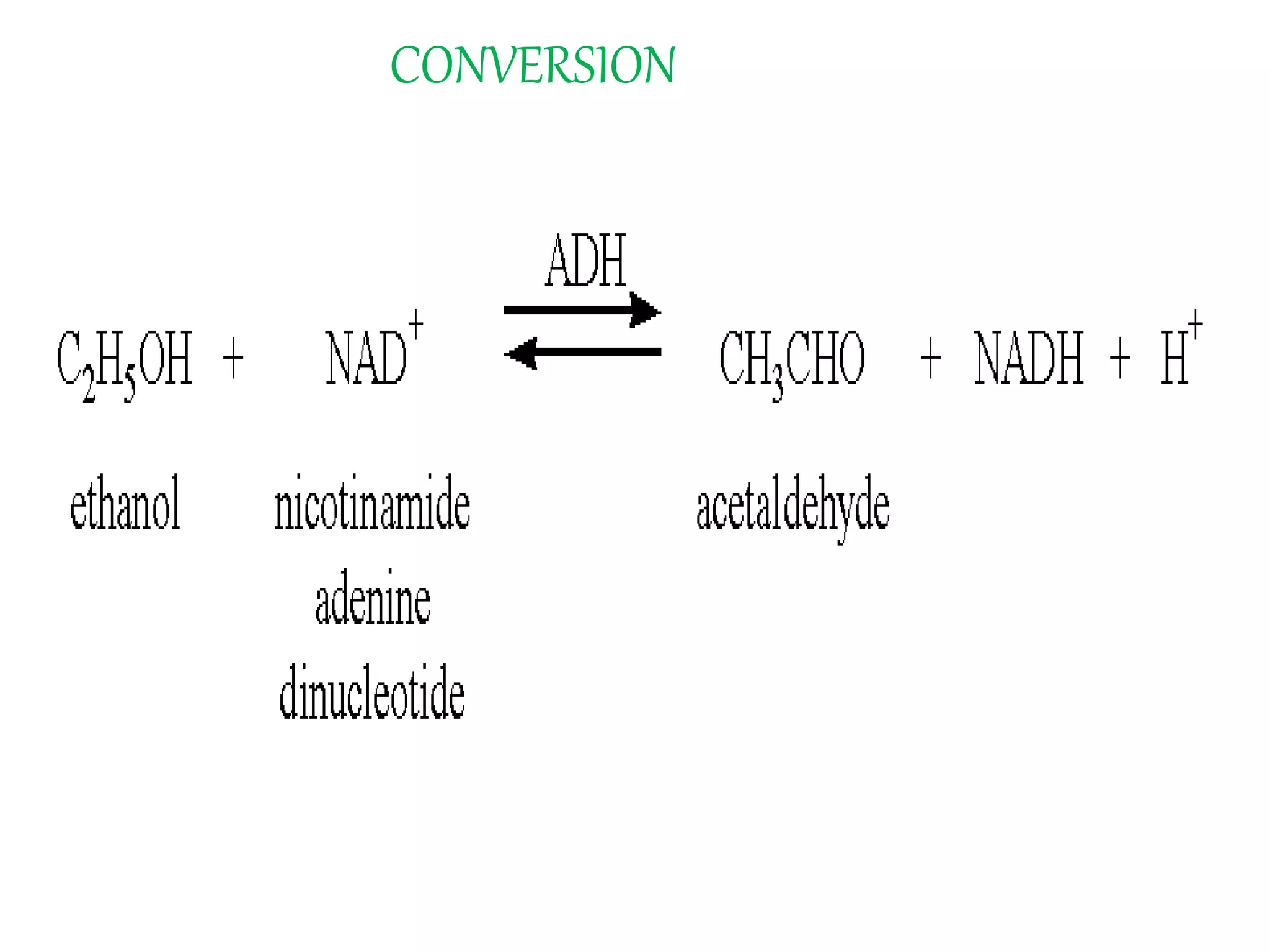
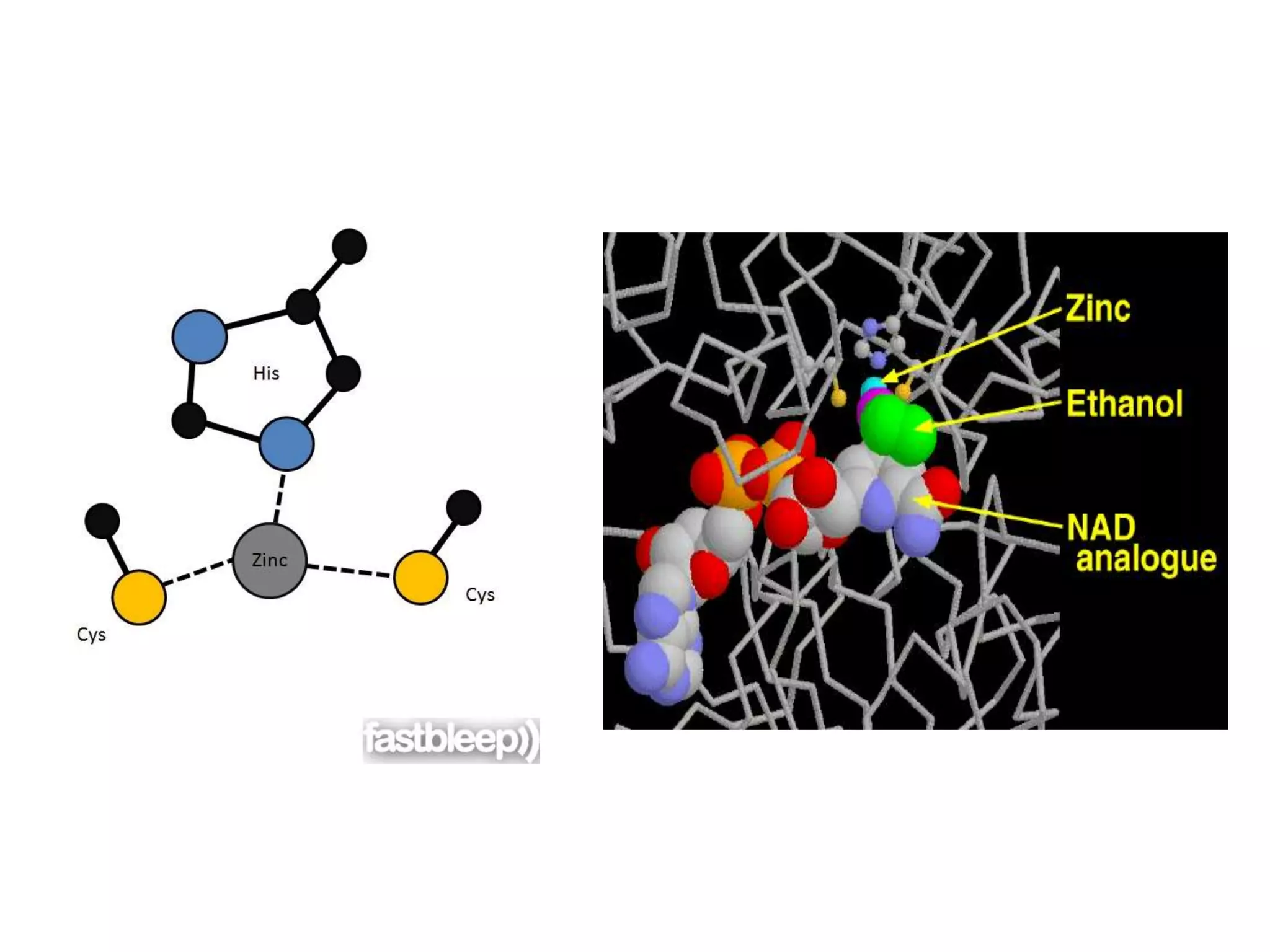
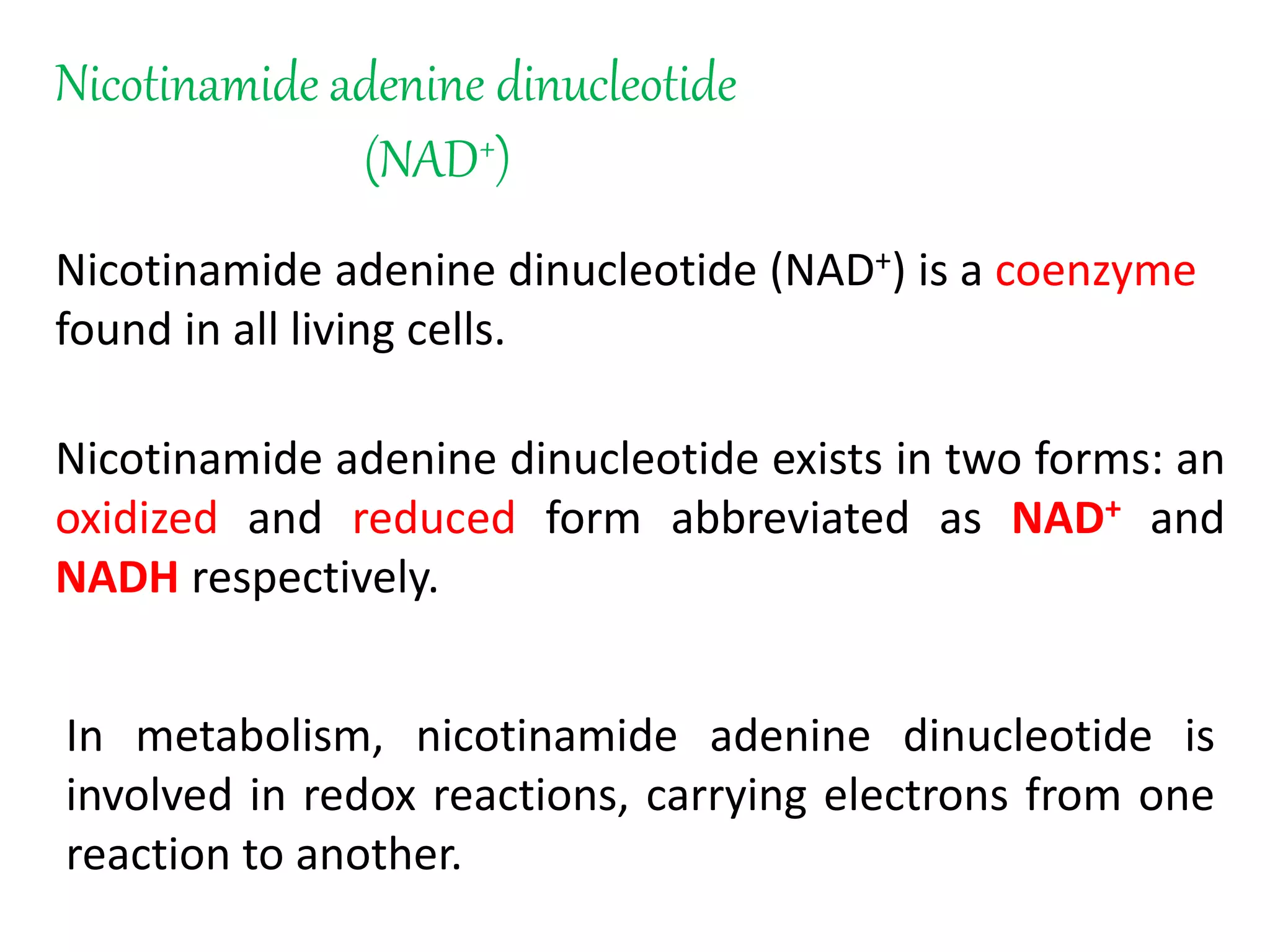
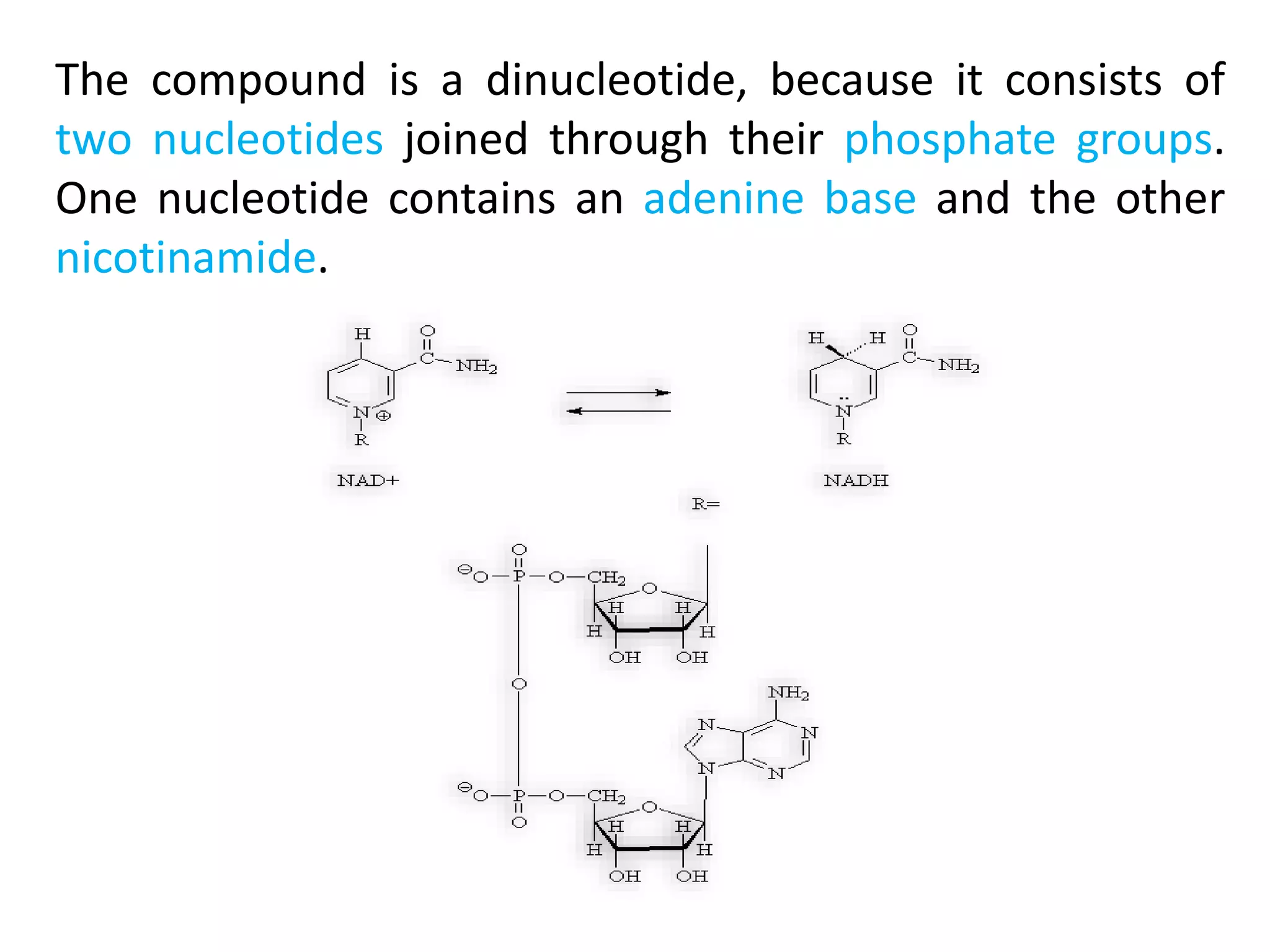
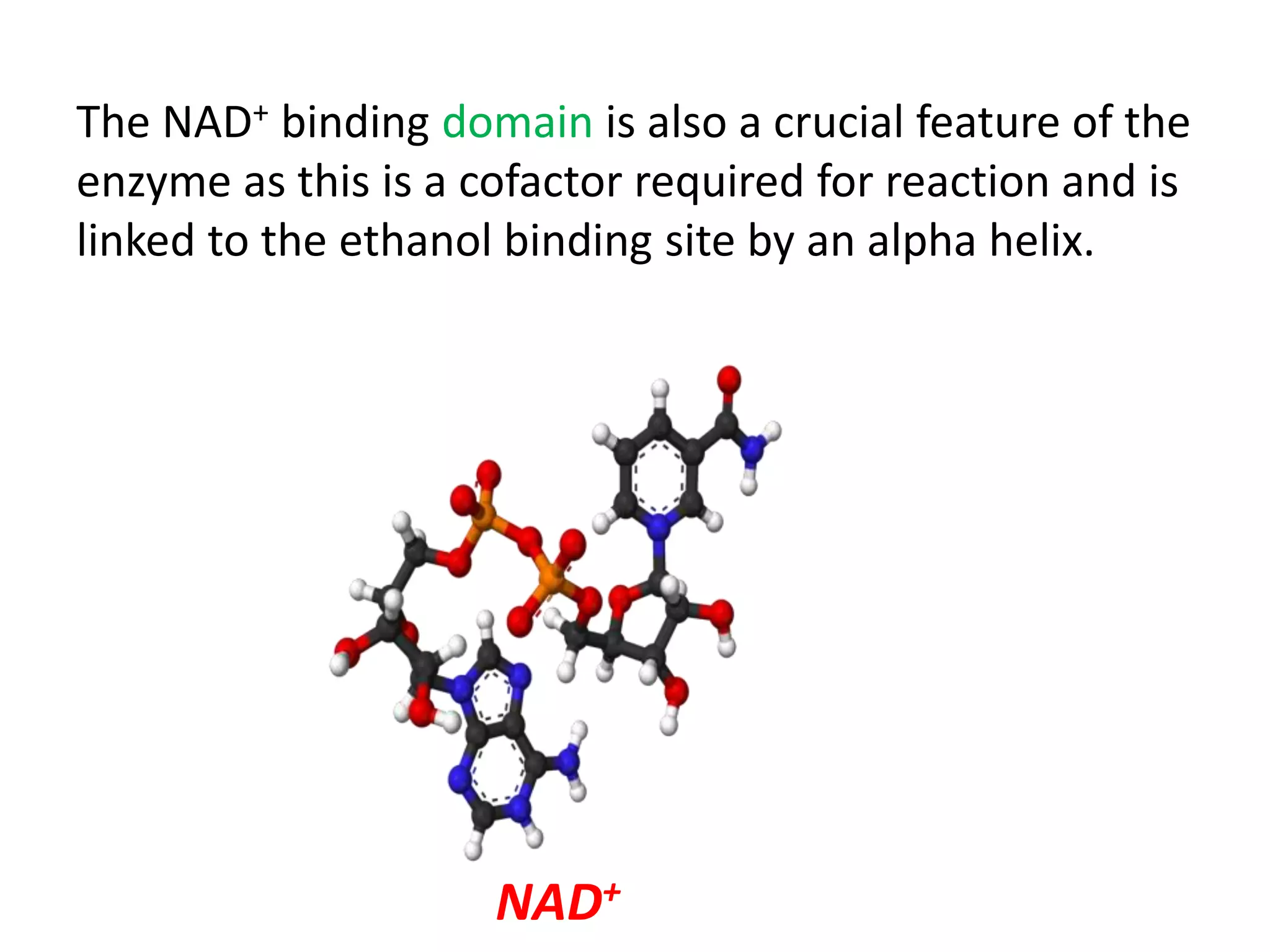
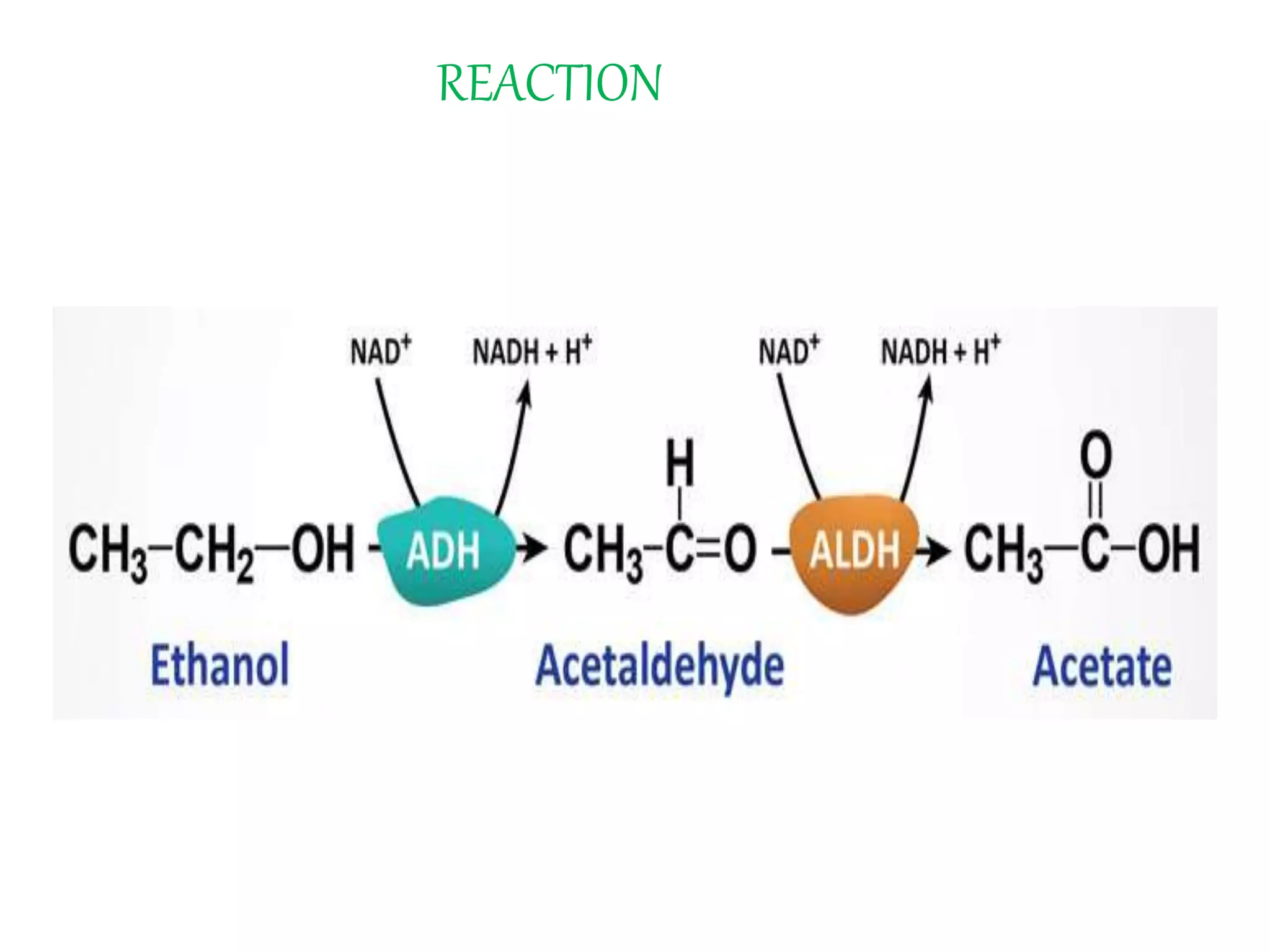
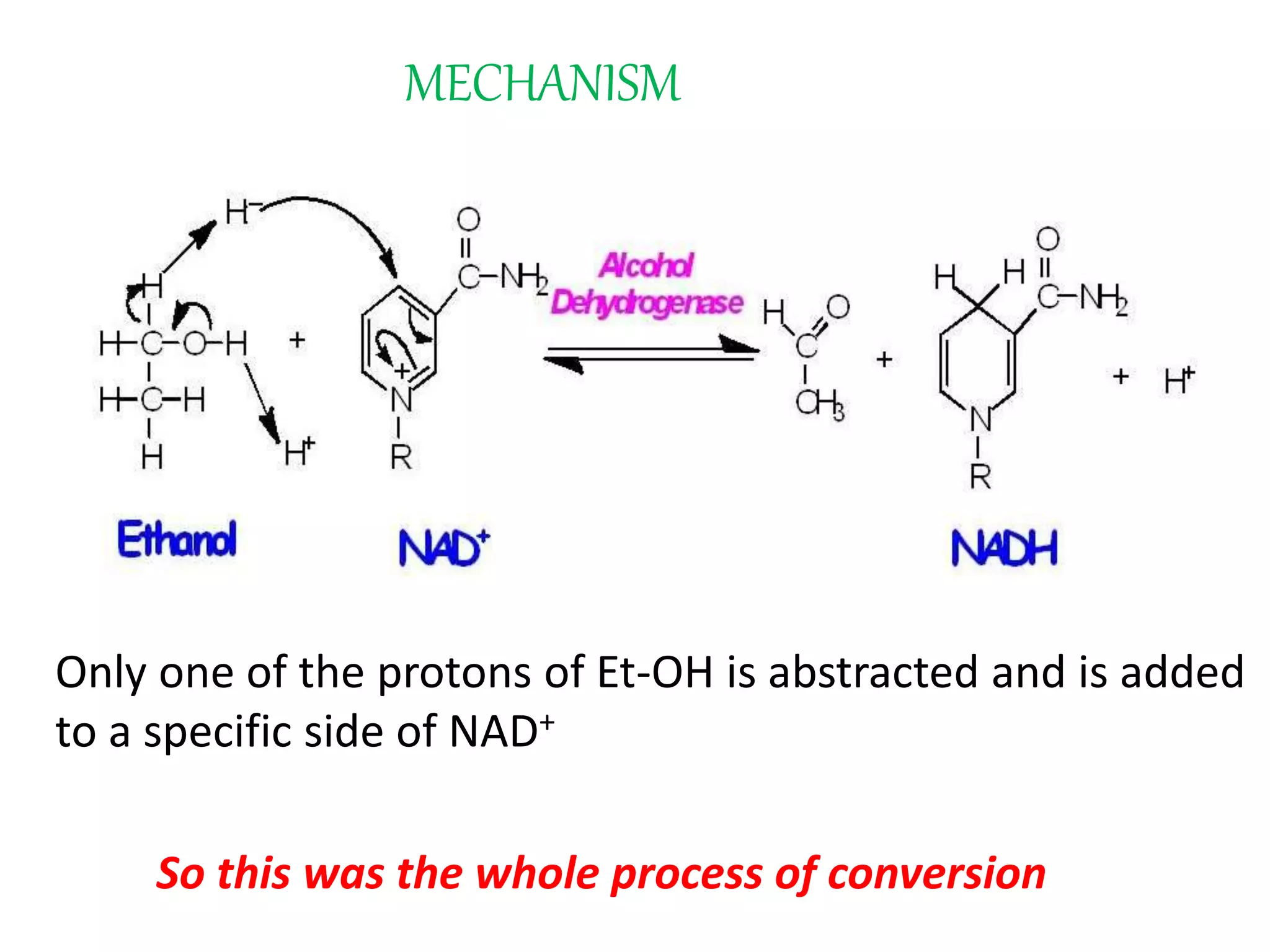
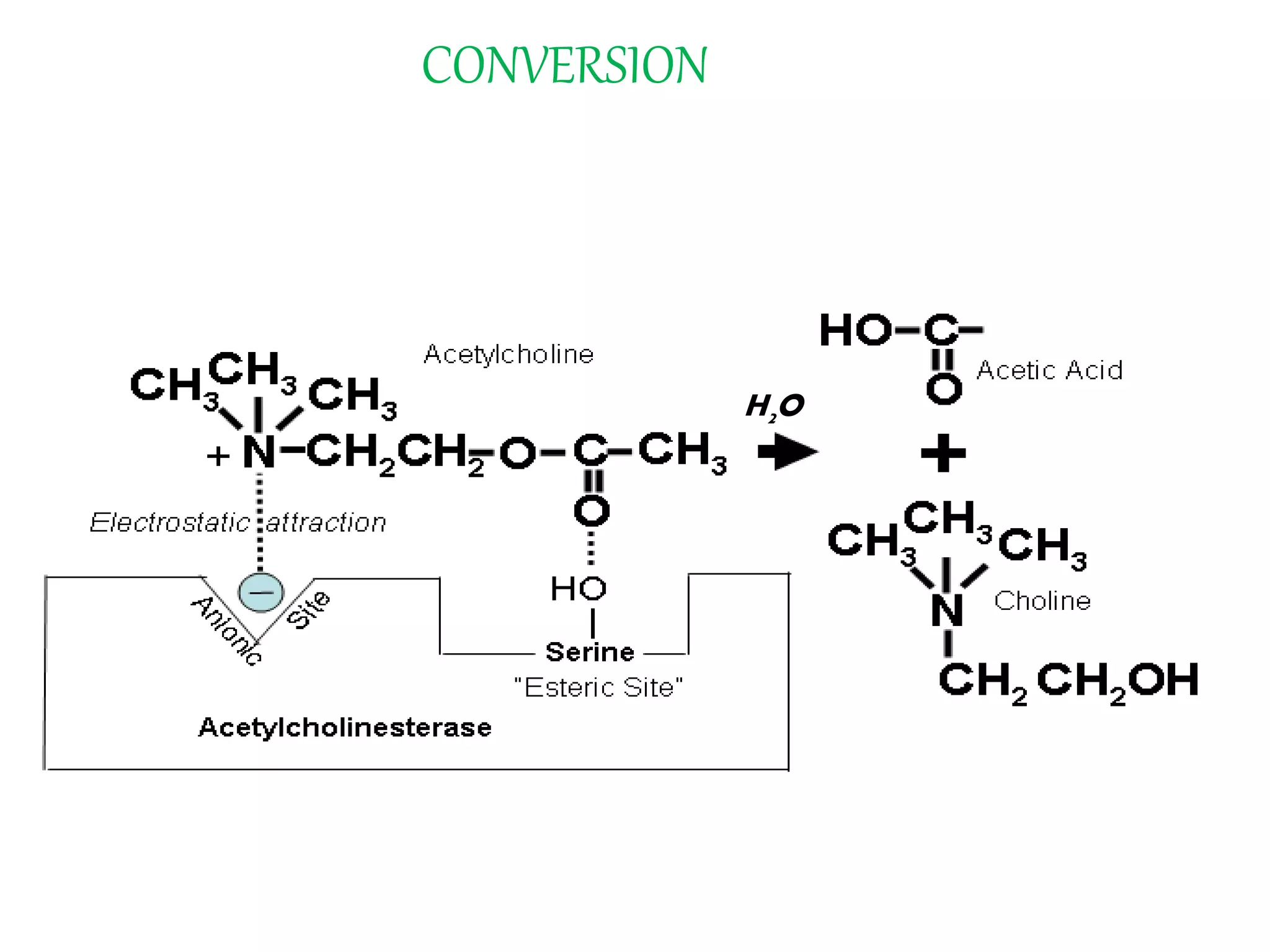
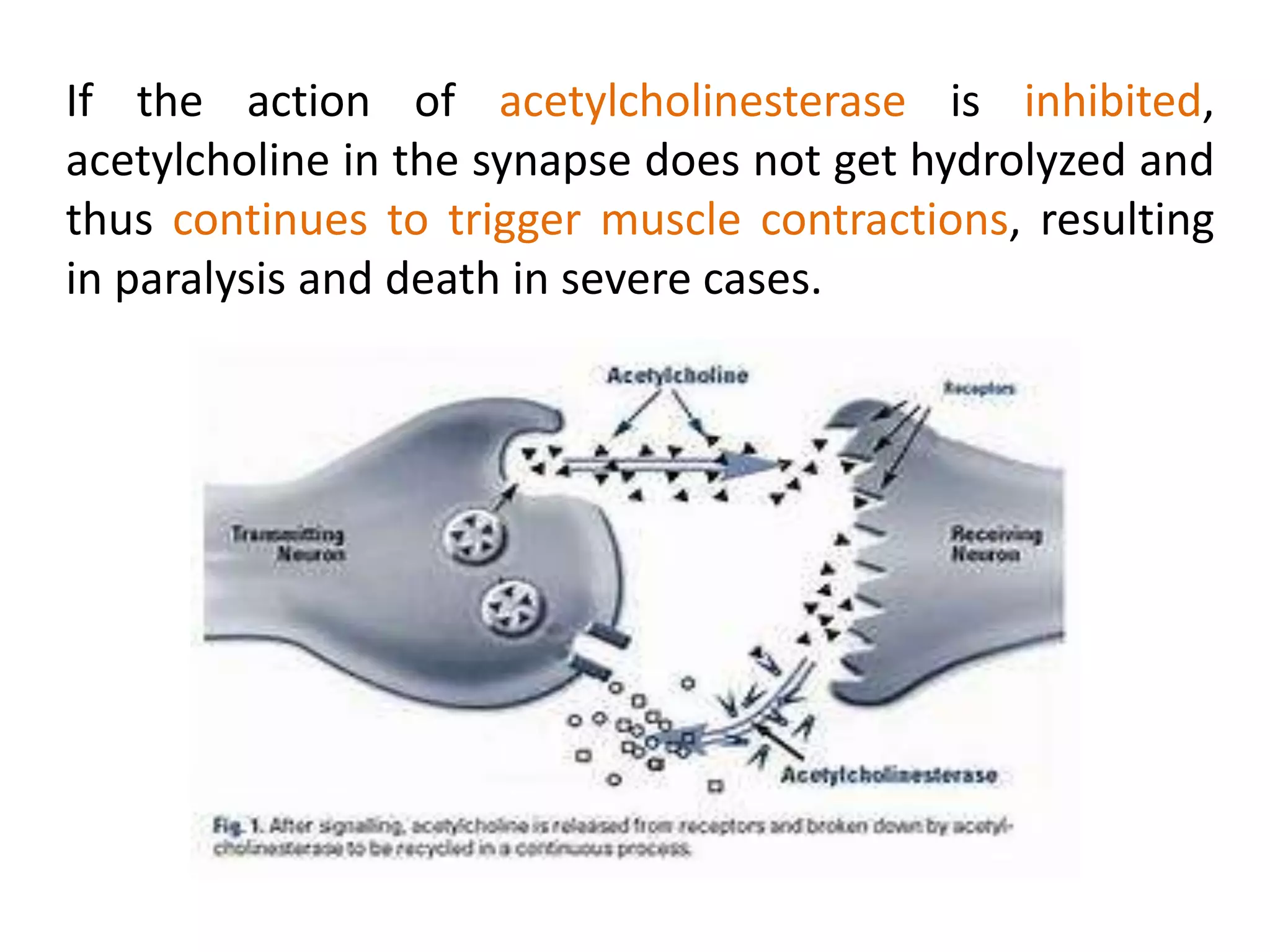
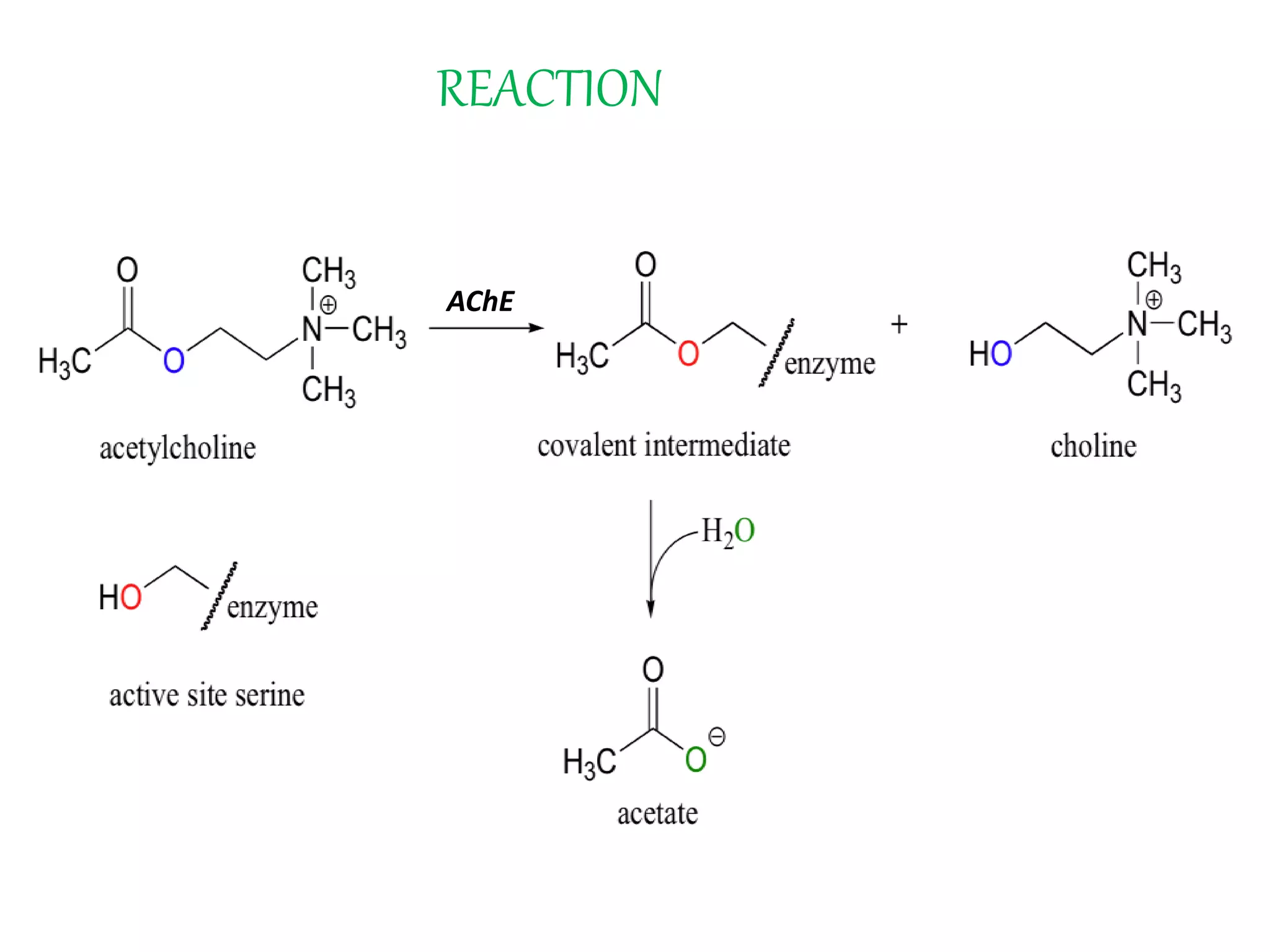
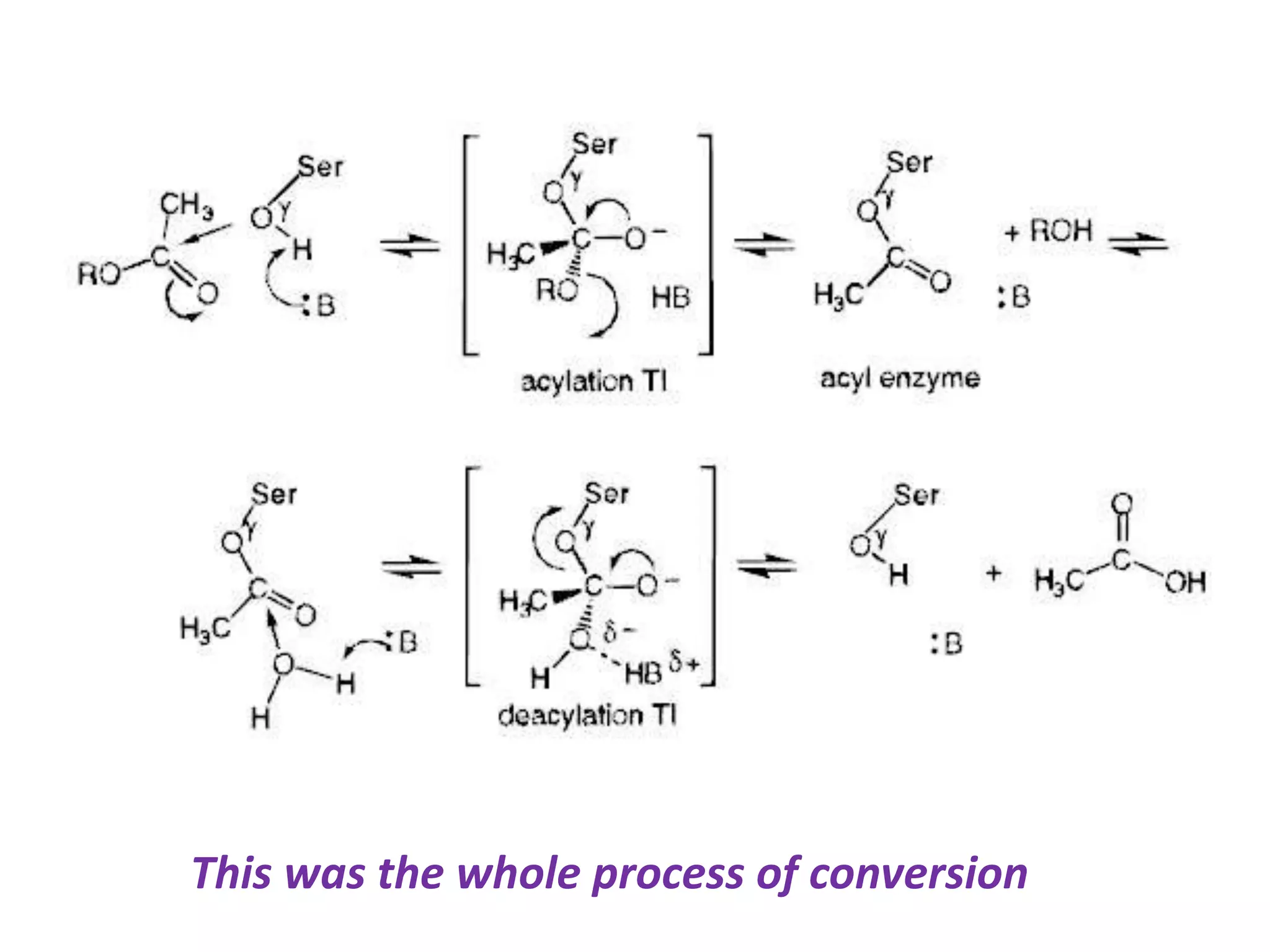
This document summarizes two enzyme-catalyzed reactions: (1) the interconversion of ethanol and acetaldehyde catalyzed by alcohol dehydrogenase, and (2) the interconversion of esters and carboxylic acids catalyzed by acetylcholinesterase. It provides details on the enzymes, cofactors, reaction mechanisms, and substrates involved in each reaction. The document was authored by Al Mamun for their M.Sc session from 2016-2017.