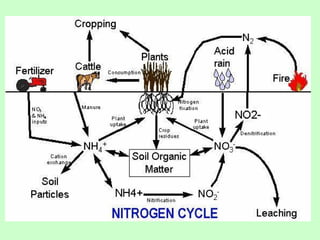
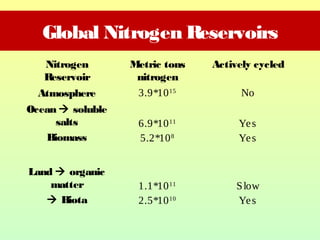
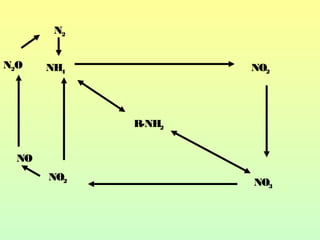
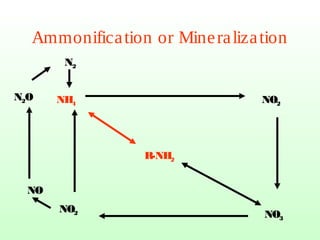
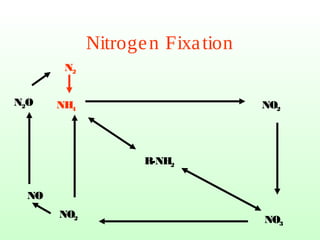
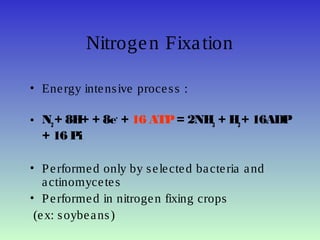
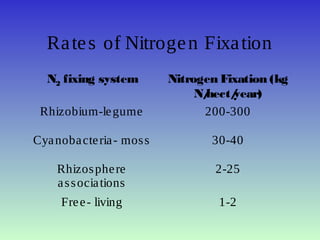
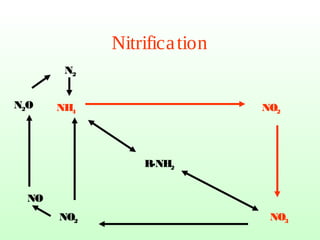
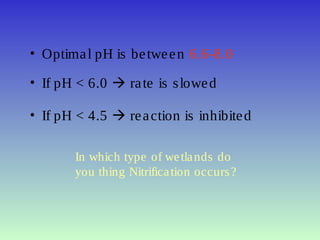
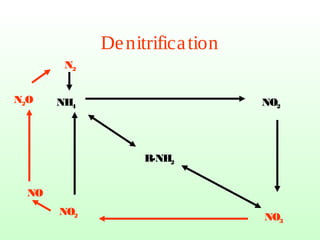
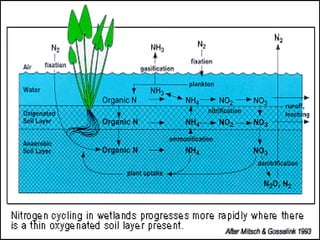
The document describes the nitrogen cycle, including its various sources, forms, reservoirs, and transformations. Key processes in the nitrogen cycle include nitrogen fixation by bacteria and cyanobacteria; ammonification and mineralization of organic nitrogen into ammonium; nitrification of ammonium to nitrite then nitrate by microbes; and denitrification of nitrates back to nitrogen gas by other microbes under anaerobic conditions. The nitrogen cycle is essential for providing nitrogen, a limiting nutrient, to living organisms and maintaining a balanced global nitrogen reservoir.
















![Surface
water
Oxidized
layer
Reduced
soil layer
[NH4]
HIGH
Low
[NH4]
Slow Diffusion
Biodegradation
C/N <20
C/N >20](https://image.slidesharecdn.com/nitrogencyclepptpresentation-171108171350/85/Nitrogen-cycle-ppt-presentation-28-320.jpg)
![Surface
water
Oxidized
layer
Reduced
soil layer
[NH4]
HIGH
Low
[NH4]
Slow Diffusion
Nitrification
[NO3] high](https://image.slidesharecdn.com/nitrogencyclepptpresentation-171108171350/85/Nitrogen-cycle-ppt-presentation-29-320.jpg)
![Surface
water
Oxidized
layer
Reduced
soil layer
[NO3] high
Leaching
[NO3] Low
N2
Denitrification](https://image.slidesharecdn.com/nitrogencyclepptpresentation-171108171350/85/Nitrogen-cycle-ppt-presentation-30-320.jpg)
