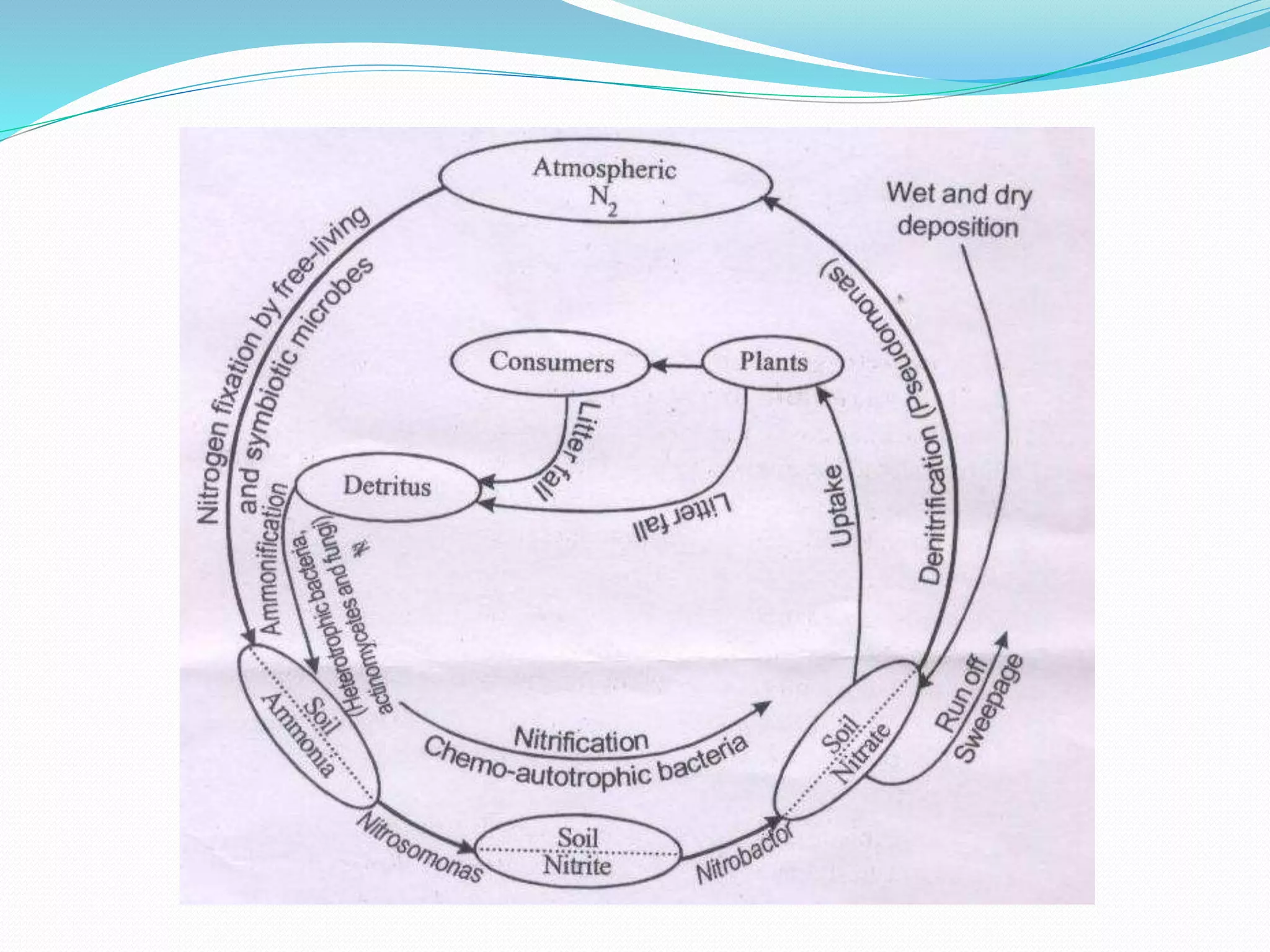
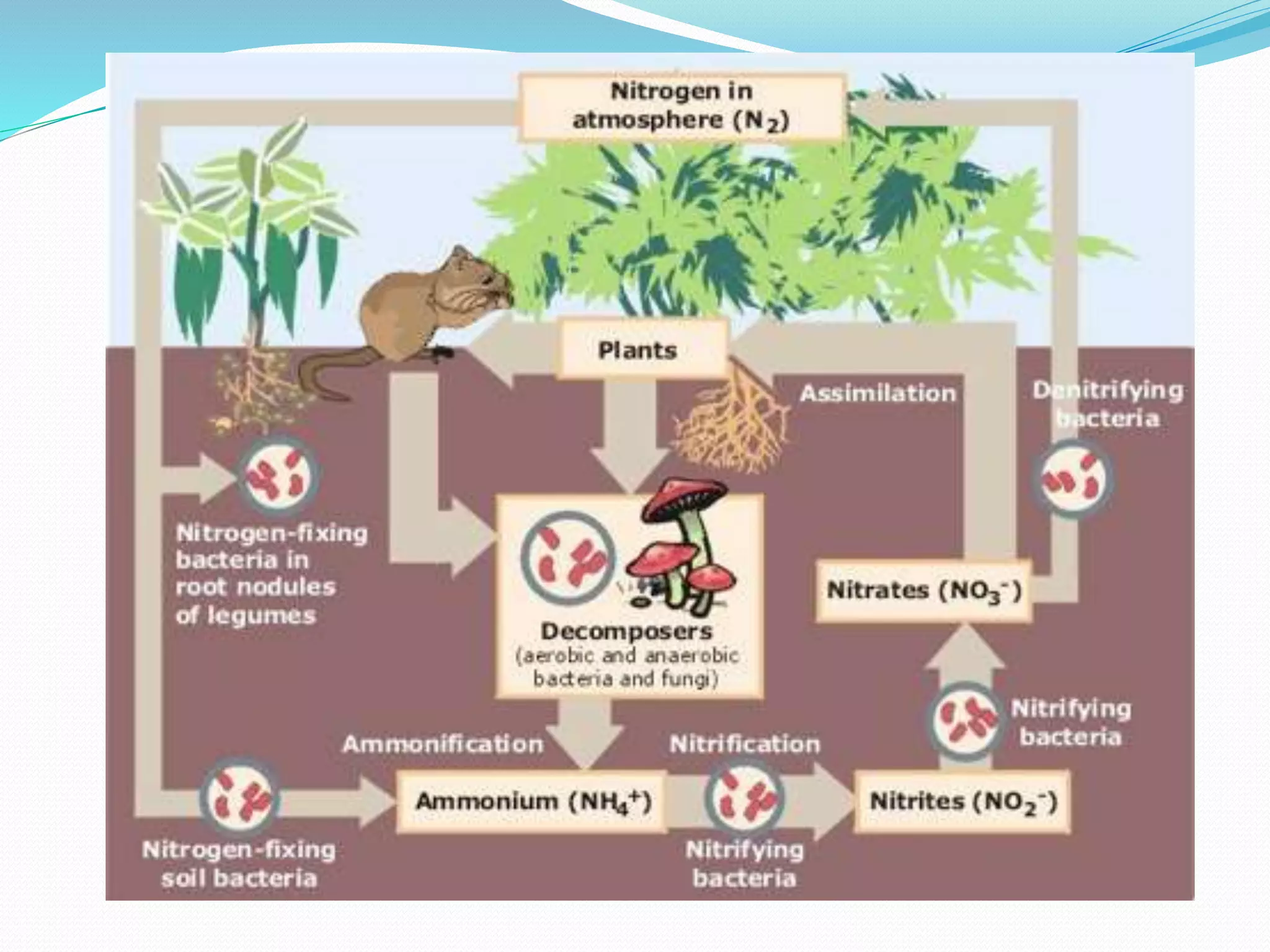
The nitrogen cycle involves the transformation of nitrogen between its various forms through biological and physical processes. Key steps include nitrogen fixation by bacteria and lightning, ammonification by microbes converting organic nitrogen to ammonia, nitrification of ammonia to nitrites then nitrates, denitrification by bacteria reducing nitrates to nitrogen gas, and assimilation of nitrates by plants. The major reservoir of nitrogen is the atmosphere, with nitrogen-fixing bacteria and cyanobacteria converting atmospheric nitrogen to ammonia or nitrates through symbiotic and non-symbiotic biological nitrogen fixation.