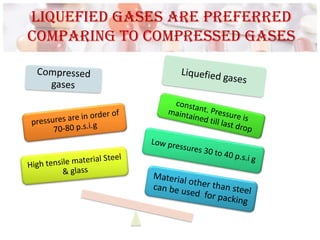
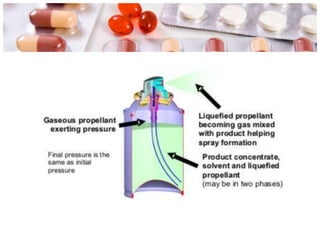
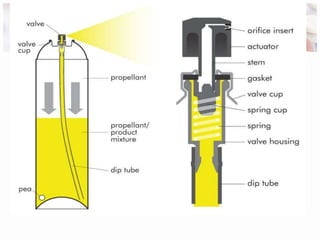
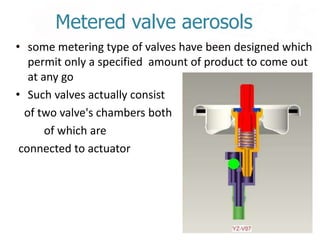
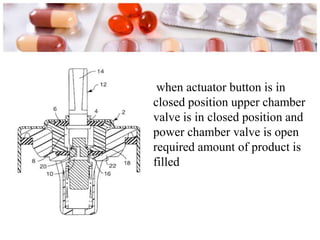
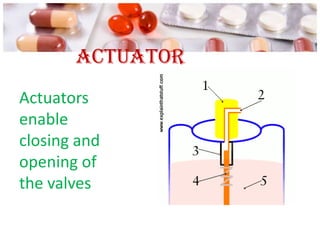
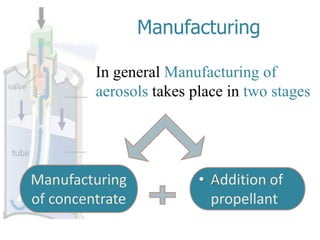
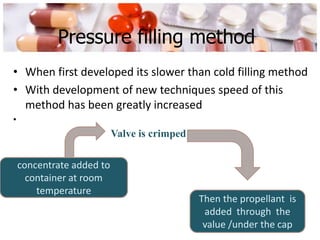
This document discusses the manufacturing and quality control of pharmaceutical aerosols. It begins by defining aerosols and their classification. It then describes the key components of an aerosol system, including propellants, containers, valves, and actuators. The two main manufacturing methods - cold filling and pressure filling - are outlined. Finally, the document details various quality control tests performed on the propellants, components, containers, and finished aerosol products to ensure quality. These include tests for leaks, spray pattern, weight, delivery rate and more.