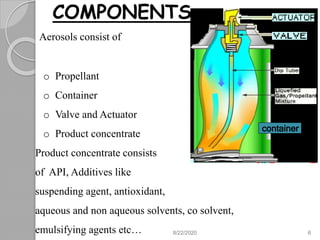
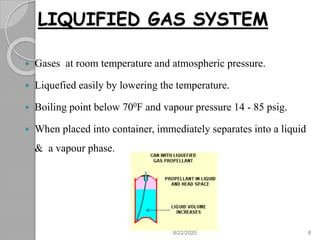
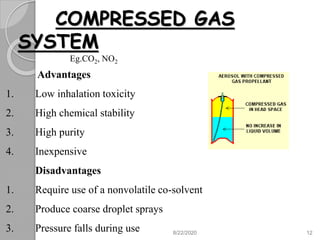
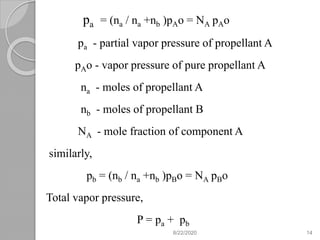
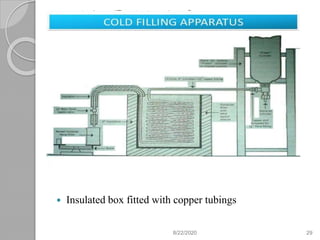
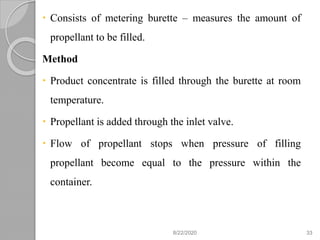
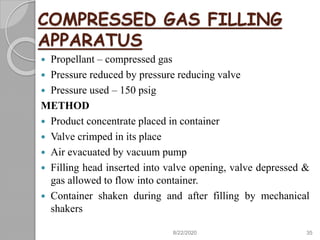
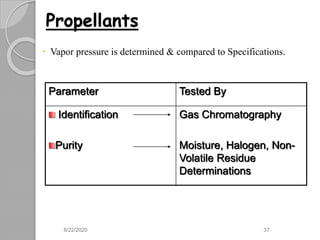
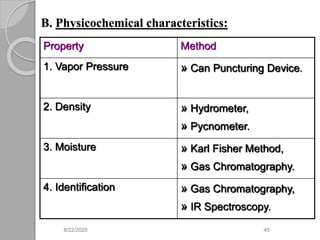
The document provides information about pharmaceutical aerosols. It discusses the history of aerosol development starting in the 1940s. It then defines aerosols and lists some of their advantages and disadvantages. The document outlines the key components of aerosols including propellants, containers, valves, and the product concentrate. It also describes the different types of propellant systems and manufacturing processes used to produce pharmaceutical aerosols. Finally, it discusses some quality control tests performed on the components and finished aerosol products.