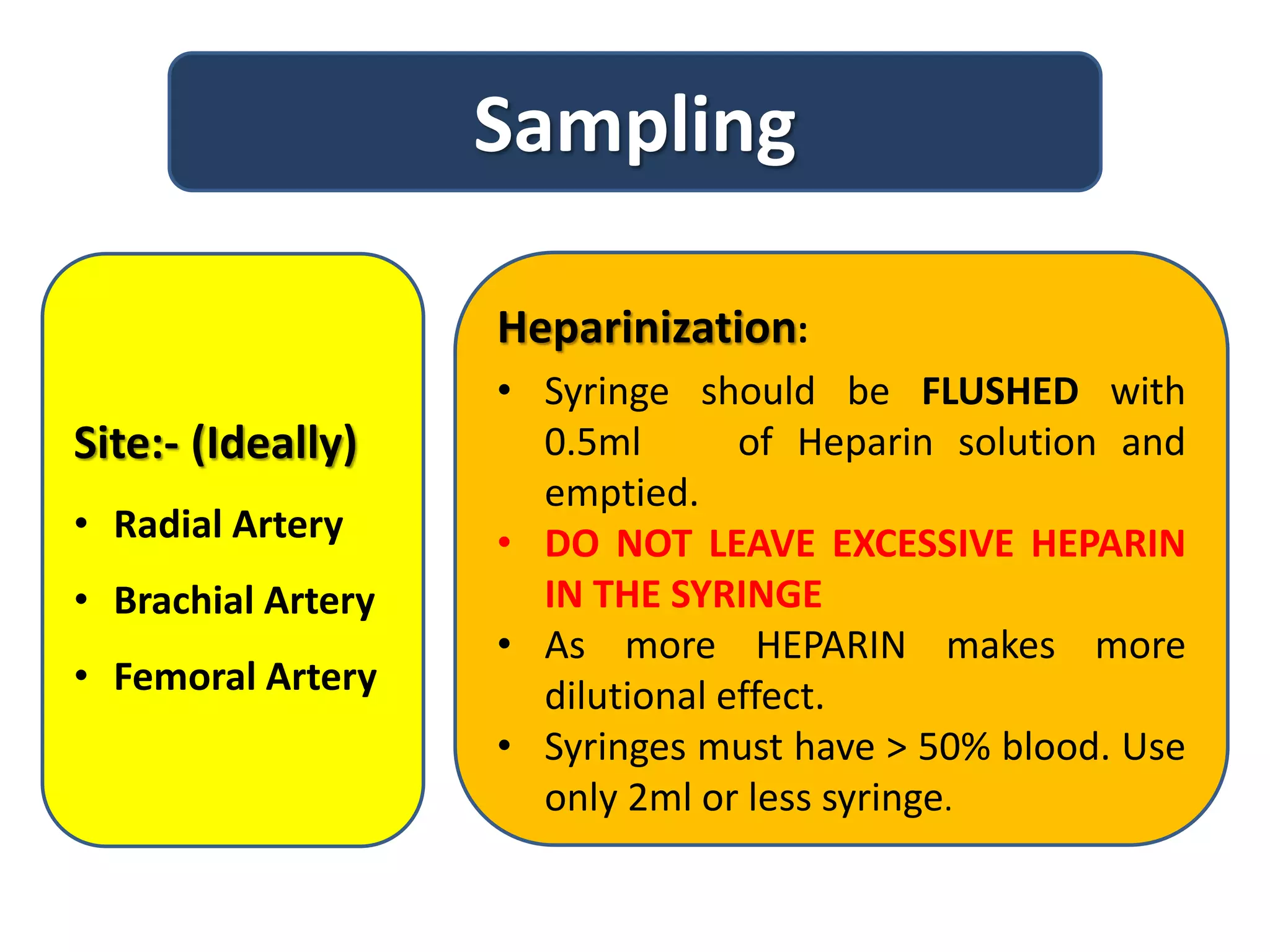
This document provides information on interpreting arterial blood gas results. It discusses sampling arterial blood properly and transporting the sample quickly for analysis. The key components measured in an ABG - pH, PaCO2, PaO2, and HCO3 - are explained. A stepwise approach is outlined to interpret ABG results to determine if a patient has a respiratory or metabolic acid-base disorder, and if it is compensated or uncompensated. Case scenarios are used to demonstrate how to classify the acid-base disorder.











![Step3: Compensated or not?
In metabolic disorders Expected PaCo2.
Metabolic acidosis:
• PCO2 = (1.5 X [HCO3
-])+8 + 2
• For every decrease by 1 in HCO3 the PCO2 falls
by 1.25 mm Hg
Metabolic alkalosis:
• PCO2 = (0.7 X [HCO3
-])+ 21 + 2
• For every increase by 1 in HCO3 the PCO2
increases by 0.75 mm Hg](https://image.slidesharecdn.com/abg-200502082850/75/ABG-interpretation-14-2048.jpg)

