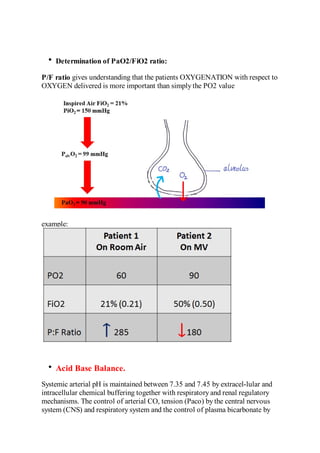
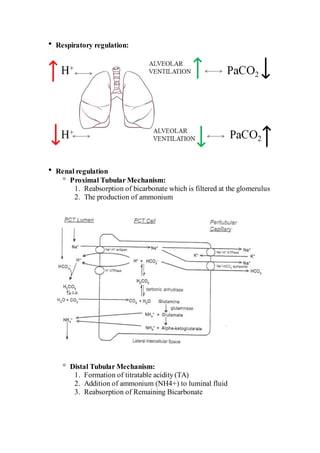
This document provides an overview of arterial blood gas (ABG) interpretation. It discusses ABG sampling procedures and indications, oxygenation and acid-base status evaluation, and a step-wise approach to ABG interpretation. It also presents examples of clinical cases and discusses metabolic and respiratory acid-base disorders and their compensatory responses.






![Metabolic disorders
Metabolic acidosis:
For every 1mmol/l decrease in HCO3 the PCO2 falls by
1.25mmHg
PCO2 = [HCO3-] + 15
Metabolic alkalosis:
For every 1mol/l increment in HCO3 the PCO2 increases
by 0.75mmHg
PCO2 = [HCO3-] + 15
Respiratory disorders:
Respiratory Acidosis:
Acute: 1 mmHg increment in PCO2 > increase in HCO3
by 0.1 meq/l
pH=7.40–0.008(PCO2-40)
Chronic: 1 mmHg increment in PCO2 > increase in HCO3
by 0.4 meq/l
pH=7.40–0.003(PCO2-40)
Respiratory alkalosis:
Acute: 1 mmHg decrease in PCO2 > decrease in HCO3 by
0.2 meq/l
pH=7.40+0.008(40-PCO2)
Chronic: 1 mmHg decrease in PCO2 > decrease in HCO3
by 0.4 meq/l
pH=7.40+0.003(40-PCO2)
Step wise approach for ABG interpretation.](https://image.slidesharecdn.com/abginterpretation-170311142613/85/Abg-interpretation-9-320.jpg)








