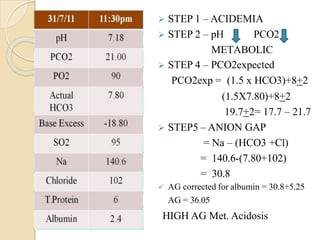
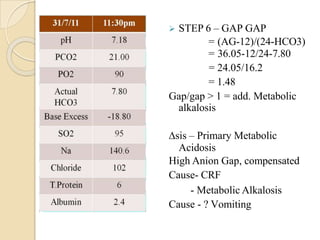
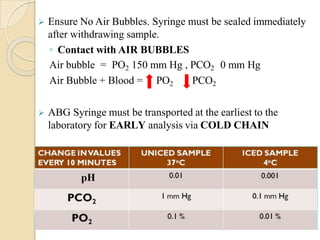
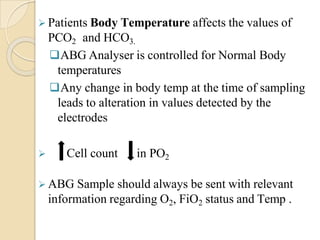
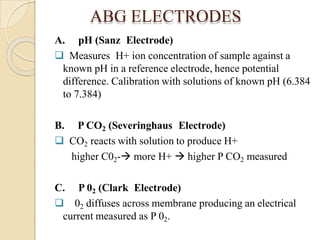
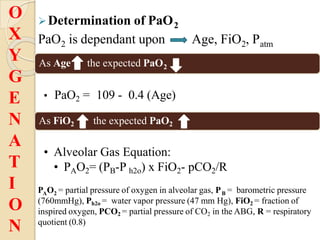
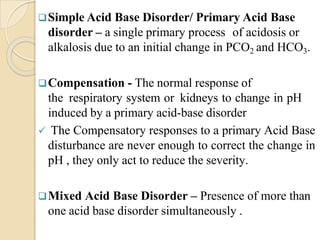
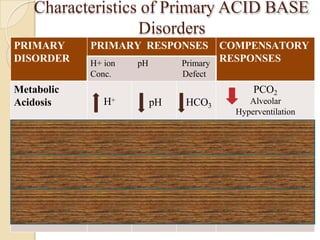
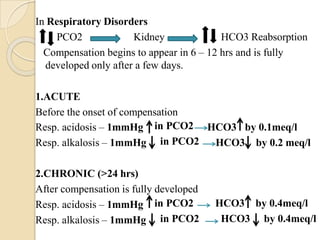
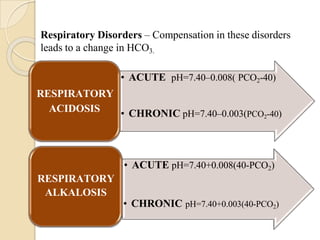
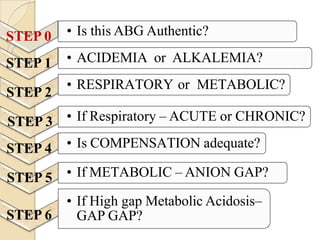
This document provides an overview of arterial blood gas interpretation. It discusses the objectives, procedure and precautions for ABG sampling. It covers the interpretation of oxygenation status including how to determine PaO2 and the PaO2/FiO2 ratio. For acid-base status, it explains the bicarbonate buffer system, respiratory and renal regulation and how to assess primary acid-base disorders. A 6-step approach to ABG interpretation is presented covering evaluating authenticity, determining acidemia/alkalemia, respiratory vs. metabolic causes, compensation and using the anion gap to identify high anion gap metabolic acidosis.


![Compensation
• PCO2 = (1.5 X [HCO3
-])+8 + 2
• PCO2 = [HCO3-] + 15
• For every 1mmol/l in HCO3 the PCO2
falls by 1.25 mm Hg
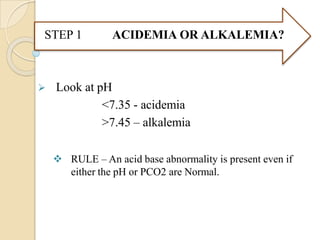
METABOLIC
ACIDOSIS
• PCO2 = (0.7 X [HCO3
-])+ 21 + 2
3
• PCO2 = [HCO -] + 15
• For every 1mol/l in HCO3 the PCO2
by 0.75 mm Hg
METABOLIC
ALKALOSIS
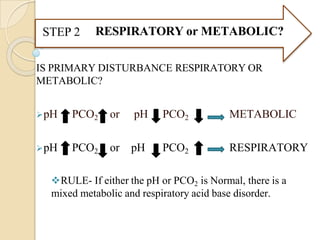
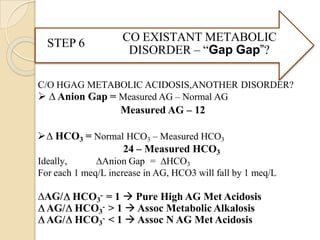
Metabolic Disorders – Compensation in these disorders leads to
a change in PCO2](https://image.slidesharecdn.com/abgobjetives-240207174337-faddc77c/85/abg-objetives-pptx-19-320.jpg)

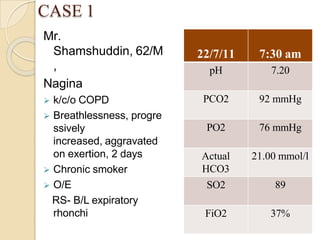
![Normal Values
ANALYTE Normal Value Units
pH 7.35 - 7.45
PCO2 35 - 45 mm Hg
PO2 72 – 104 mm Hg`
[HCO3] 22 – 30 meq/L
SaO2 95-100 %
Anion Gap 12 + 4 meq/L
∆HCO3 +2 to -2 meq/L](https://image.slidesharecdn.com/abgobjetives-240207174337-faddc77c/85/abg-objetives-pptx-23-320.jpg)
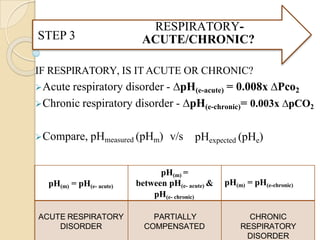
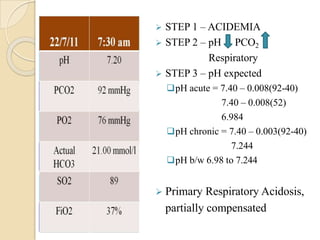
![Is this ABG authentic ?
⚫ pH = - log [H+]
Henderson-Hasselbalch equation
pH = 6.1 + log HCO3
-
0.03 x PCO2
The [HCO3-] mentioned on the ABG is actually calculated
using this equation from measured values of PCO2 nd pH
• [H+] neq/l = 24 X (PCO2 / HCO3)
• pH = -log [ H+]
pHexpected = pHmeasured = ABG is authentic](https://image.slidesharecdn.com/abgobjetives-240207174337-faddc77c/85/abg-objetives-pptx-25-320.jpg)
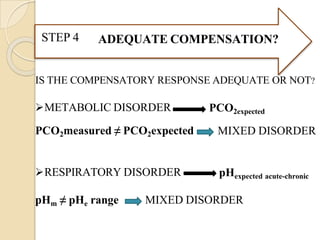
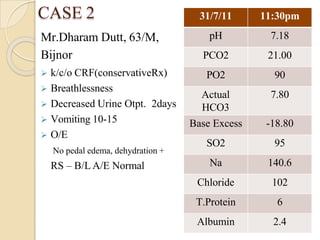
![⚫Reference table for pH v/s [H+]
[H+] neq/l = 24 X (PCO2 / HCO3)
H+ ion pH
100 7.00
79 7.10
63 7.20
50 7.30
45 7.35
40 7.40
35 7.45
32 7.50
25 7.60](https://image.slidesharecdn.com/abgobjetives-240207174337-faddc77c/85/abg-objetives-pptx-26-320.jpg)