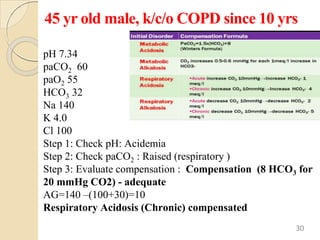
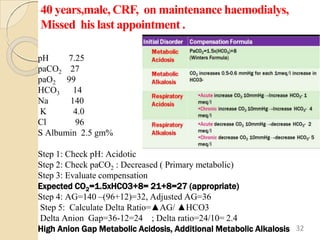
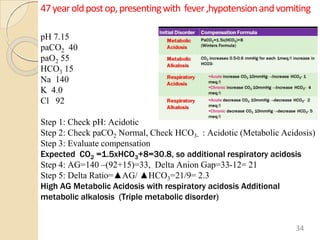
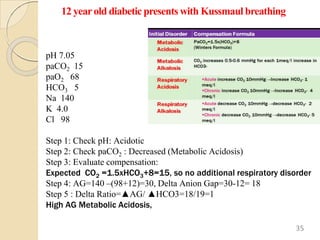
The document presents a comprehensive guide on arterial blood gas (ABG) interpretation by Dr. Aman Jain, covering the preanalytical phase, ABG procedure, and detailed steps for evaluating acid-base balance. It outlines key definitions, compensation mechanisms, and provides a systematic approach to assess various acid-base disorders using specific equations. Additionally, the document includes numerous clinical case scenarios illustrating the principles of ABG interpretation.



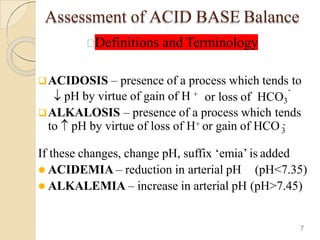
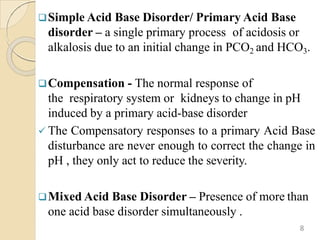

![Normal Values
ANALYTE Normal Value Units
pH 7.35 - 7.45
PCO2 35 - 45 mm Hg
PO2 72 – 104 mm Hg`
[HCO3] 22 – 30 meq/L
SaO2 95-100 %
Anion Gap 12 + 4 meq/L
∆HCO3 +2 to -2 meq/L
10](https://image.slidesharecdn.com/abg-dr-240429095401-f3d2500a/85/ABG-analysis-presentation-by-Dr-Aman-jain-10-320.jpg)

![Is this ABG authentic ?
pH = - log [H+]
Henderson-Hasselbalch equation
pH = 6.1 + log HCO3
-
0.03 x PCO2
The [HCO3-] mentioned on the ABG is actually calculated
using this equation from measured values of PCO2 ndpH
• [H+] neq/l = 24 X (PCO2 / HCO3)
• pH = -log [ H+]
pHexpected = pHmeasured = ABG is authentic
12](https://image.slidesharecdn.com/abg-dr-240429095401-f3d2500a/85/ABG-analysis-presentation-by-Dr-Aman-jain-12-320.jpg)
![Reference table for pH v/s [H+]
[H+] neq/l = 24 X (PCO2 /HCO3)
H+ ion pH
100 7.00
79 7.10
63 7.20
50 7.30
45 7.35
40 7.40
35 7.45
32 7.50
25 7.60
13](https://image.slidesharecdn.com/abg-dr-240429095401-f3d2500a/85/ABG-analysis-presentation-by-Dr-Aman-jain-13-320.jpg)