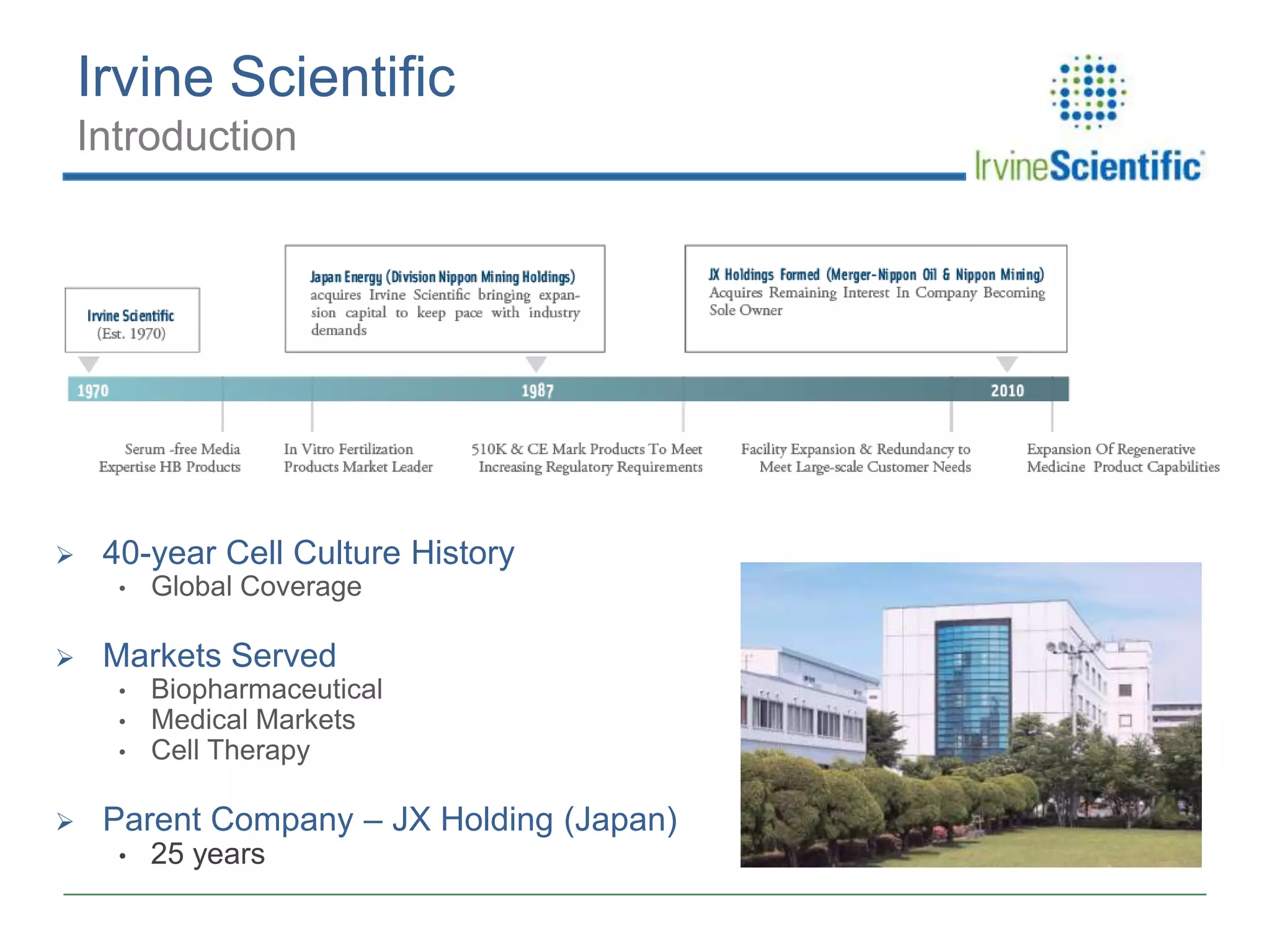
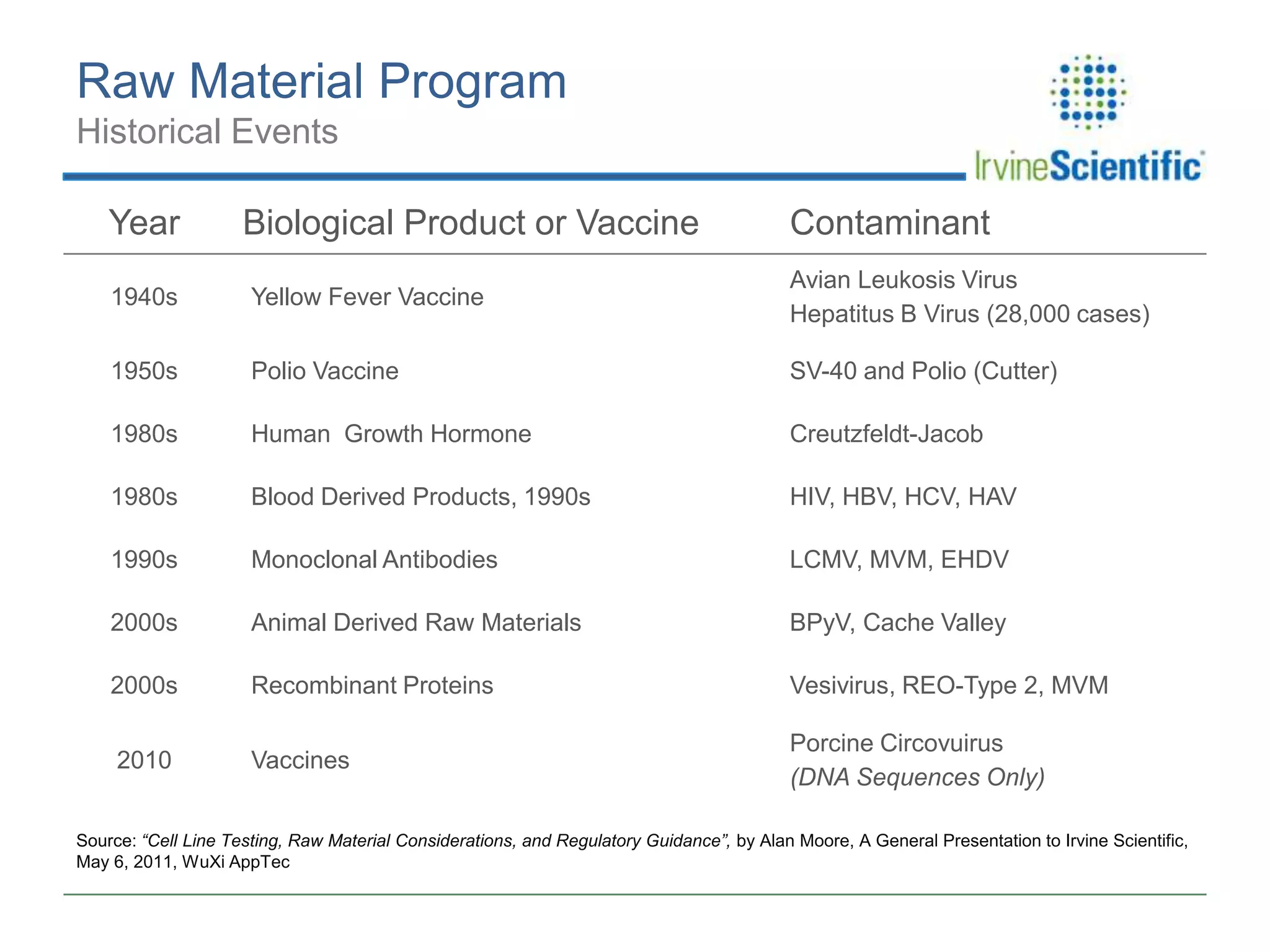
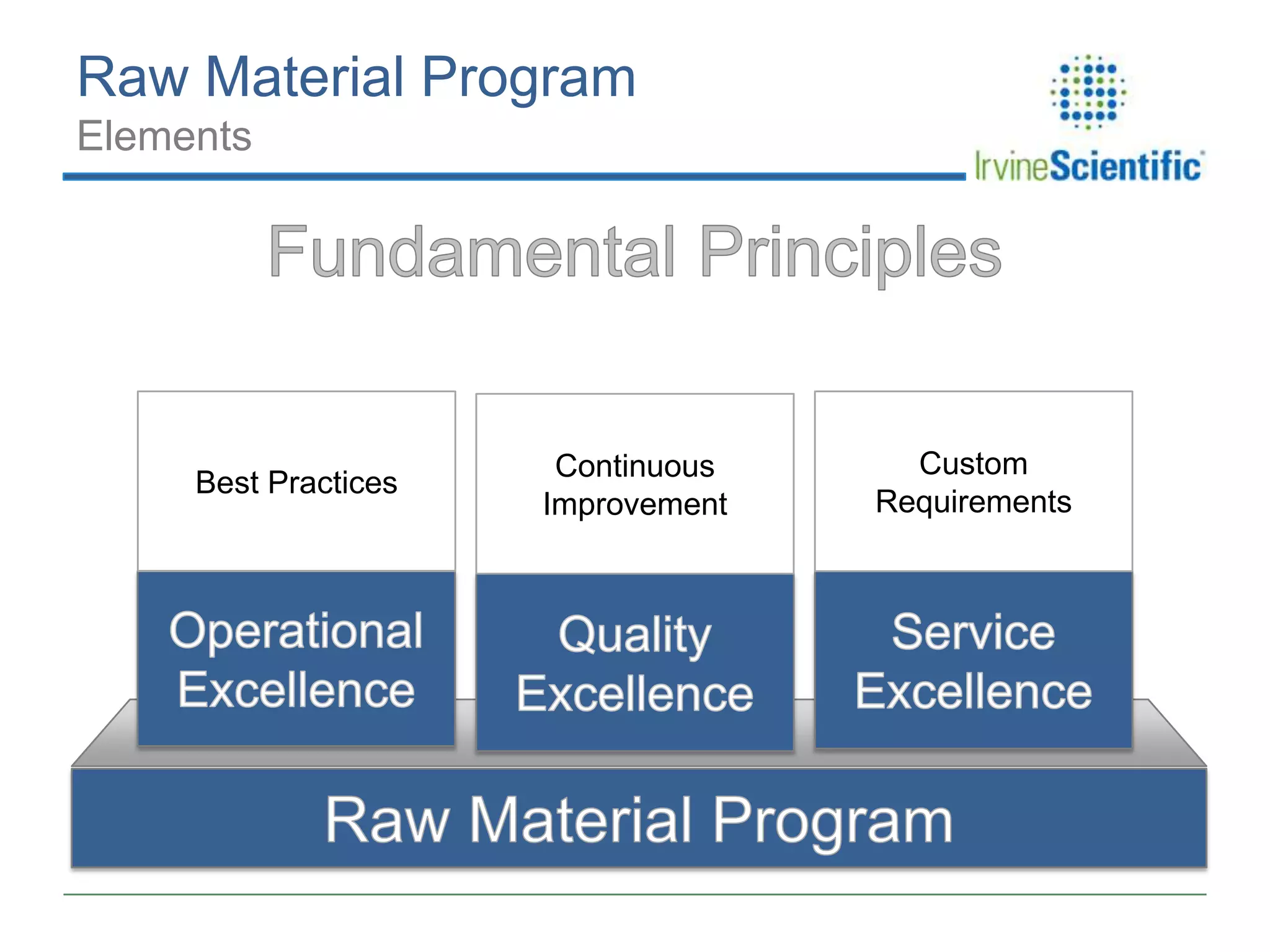
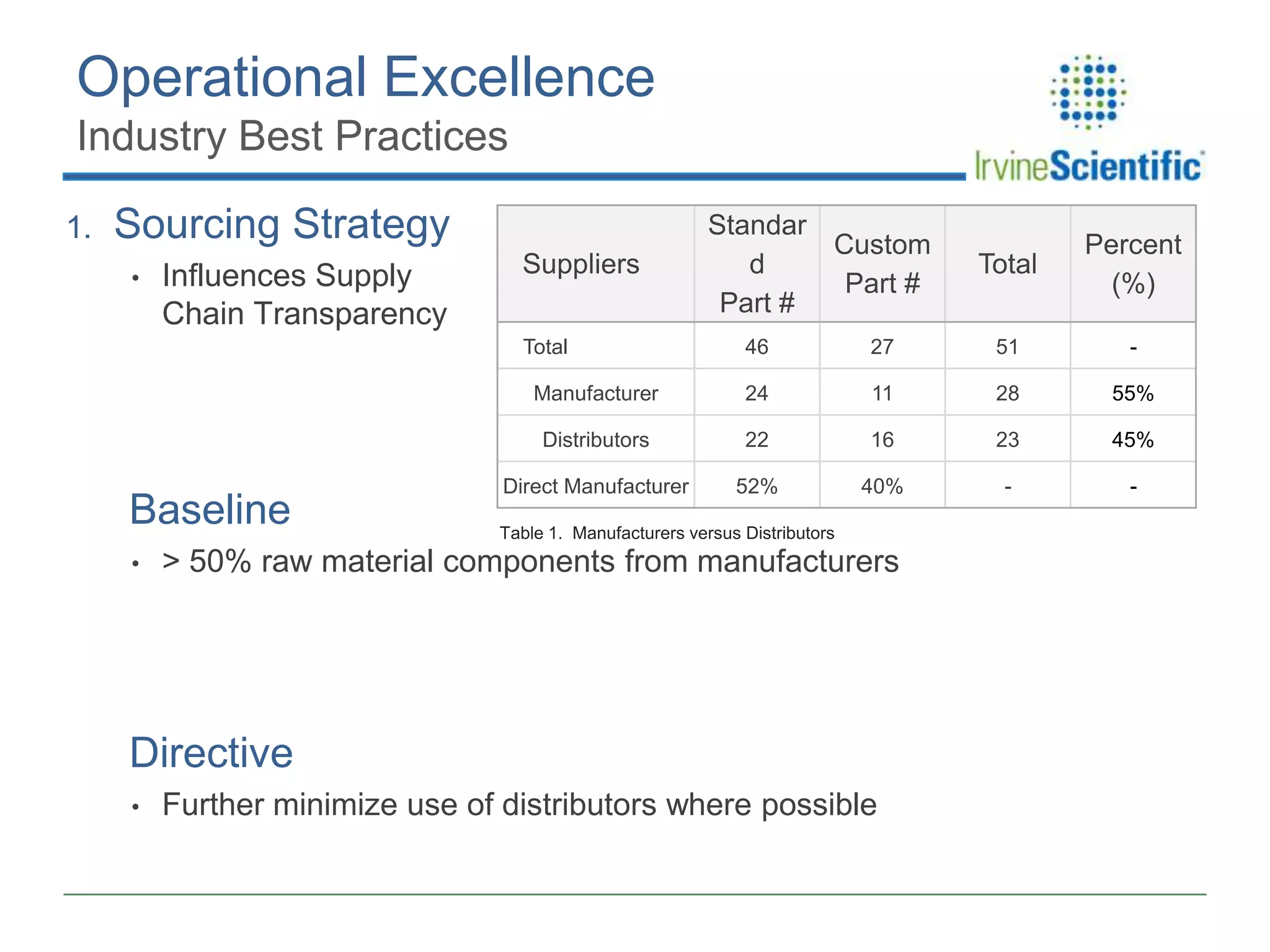
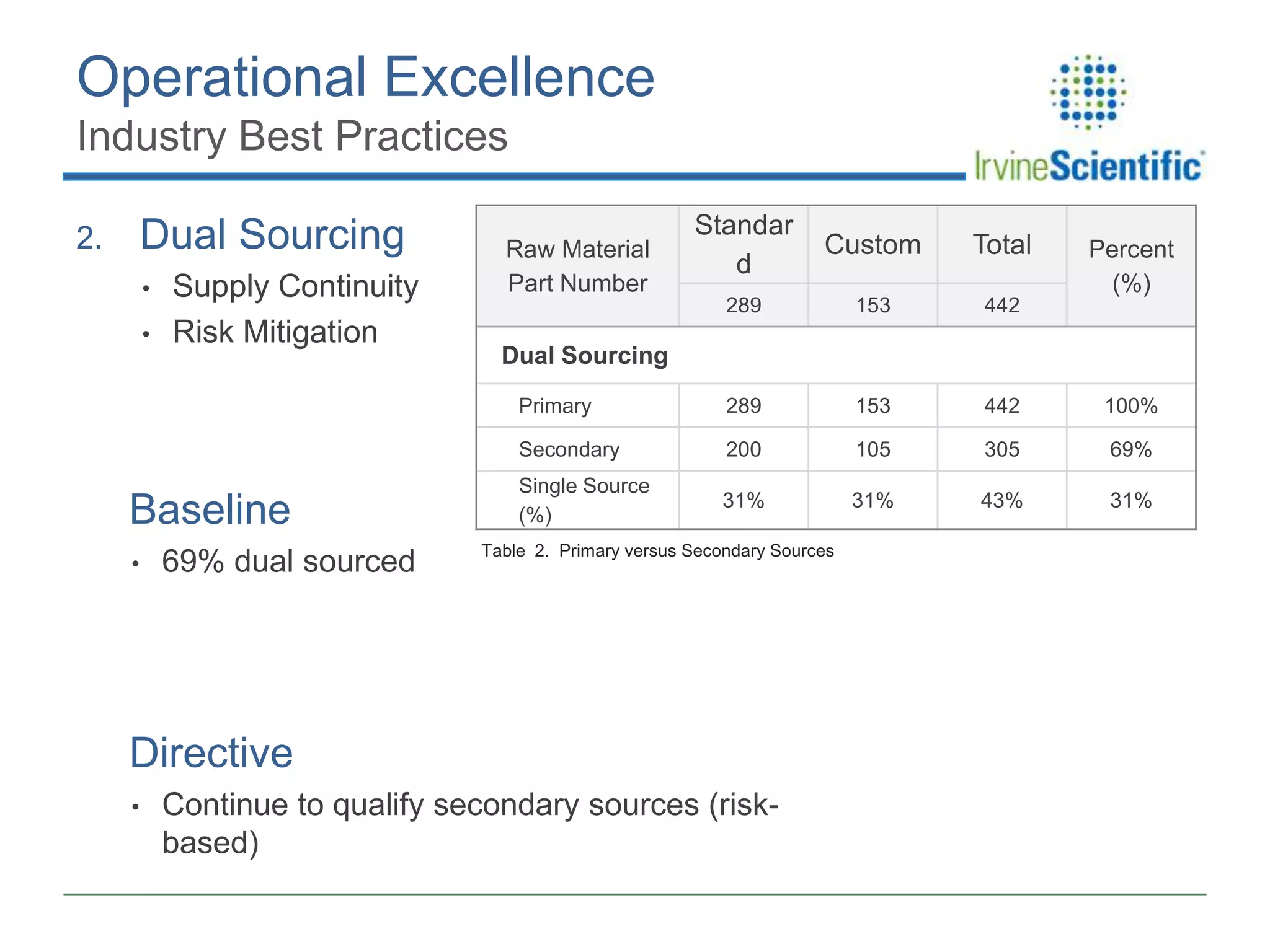
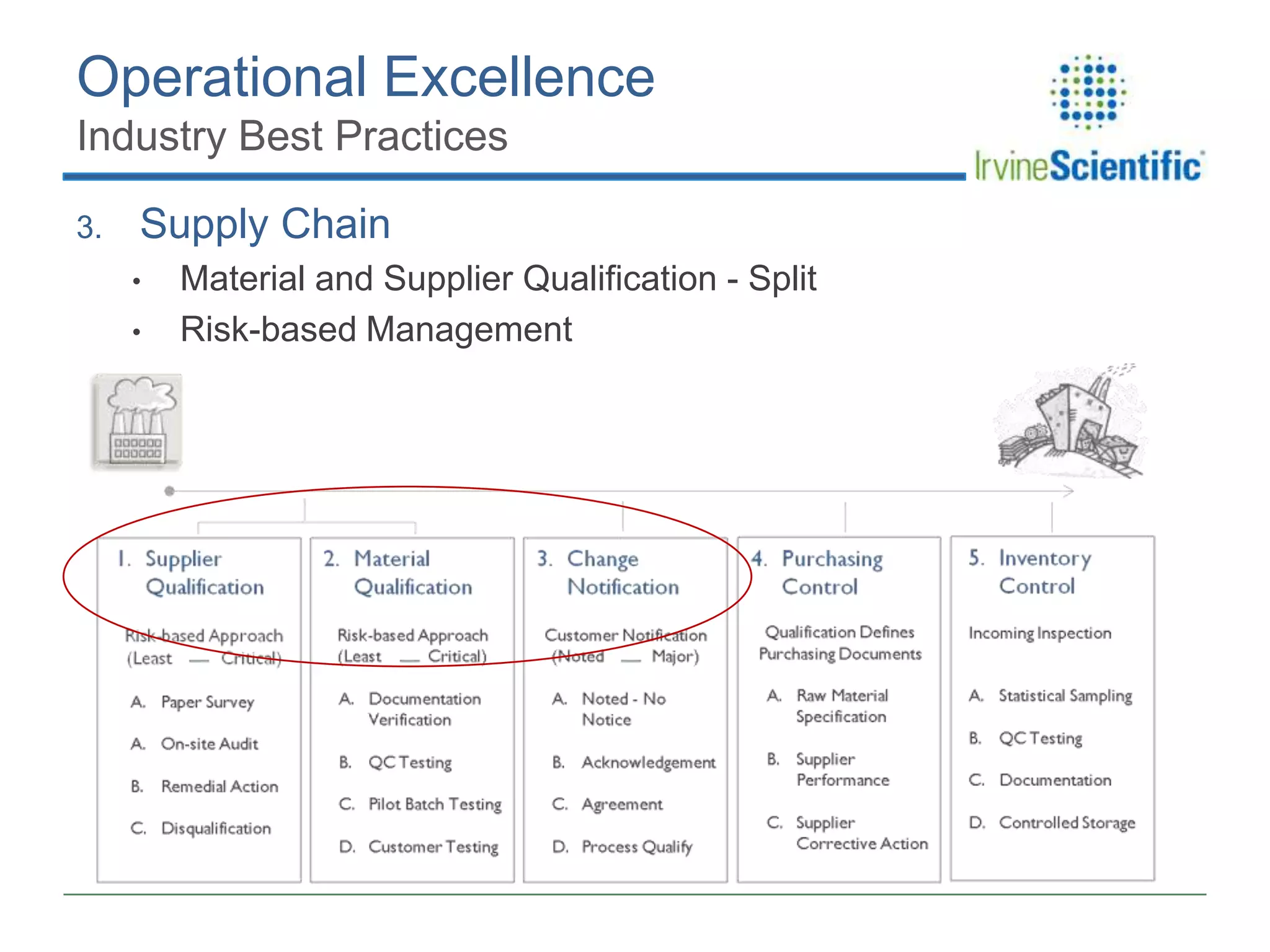
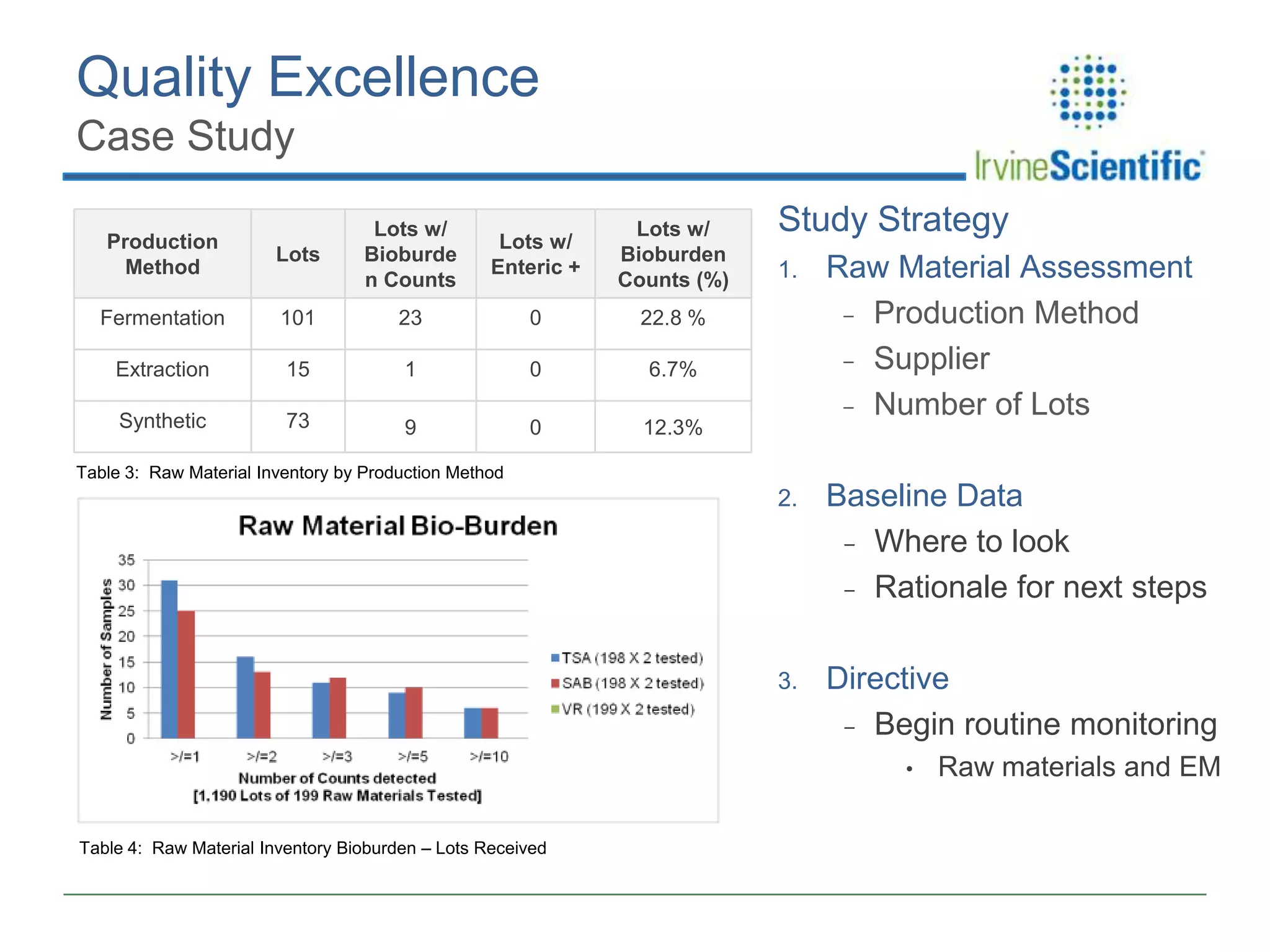
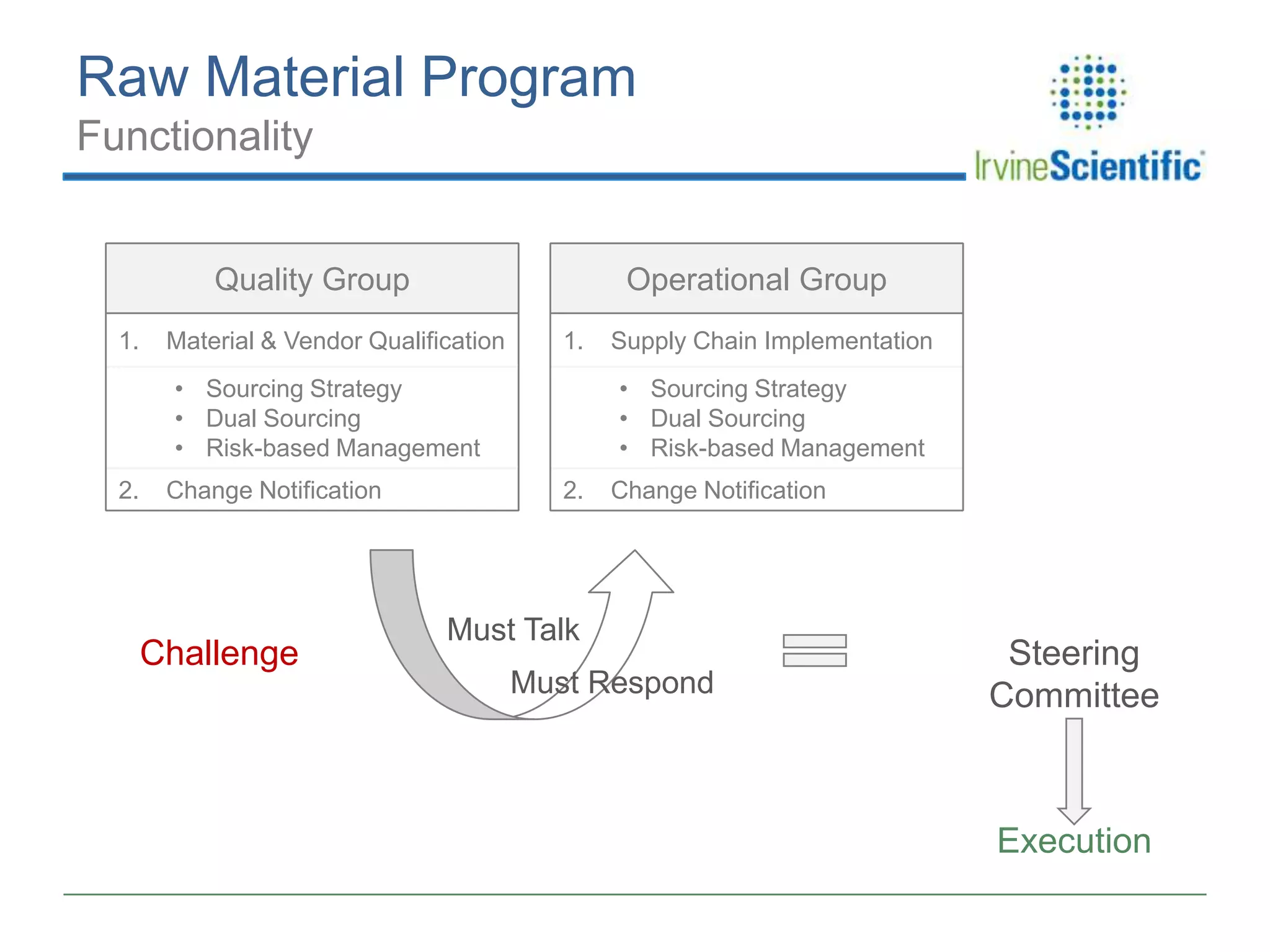
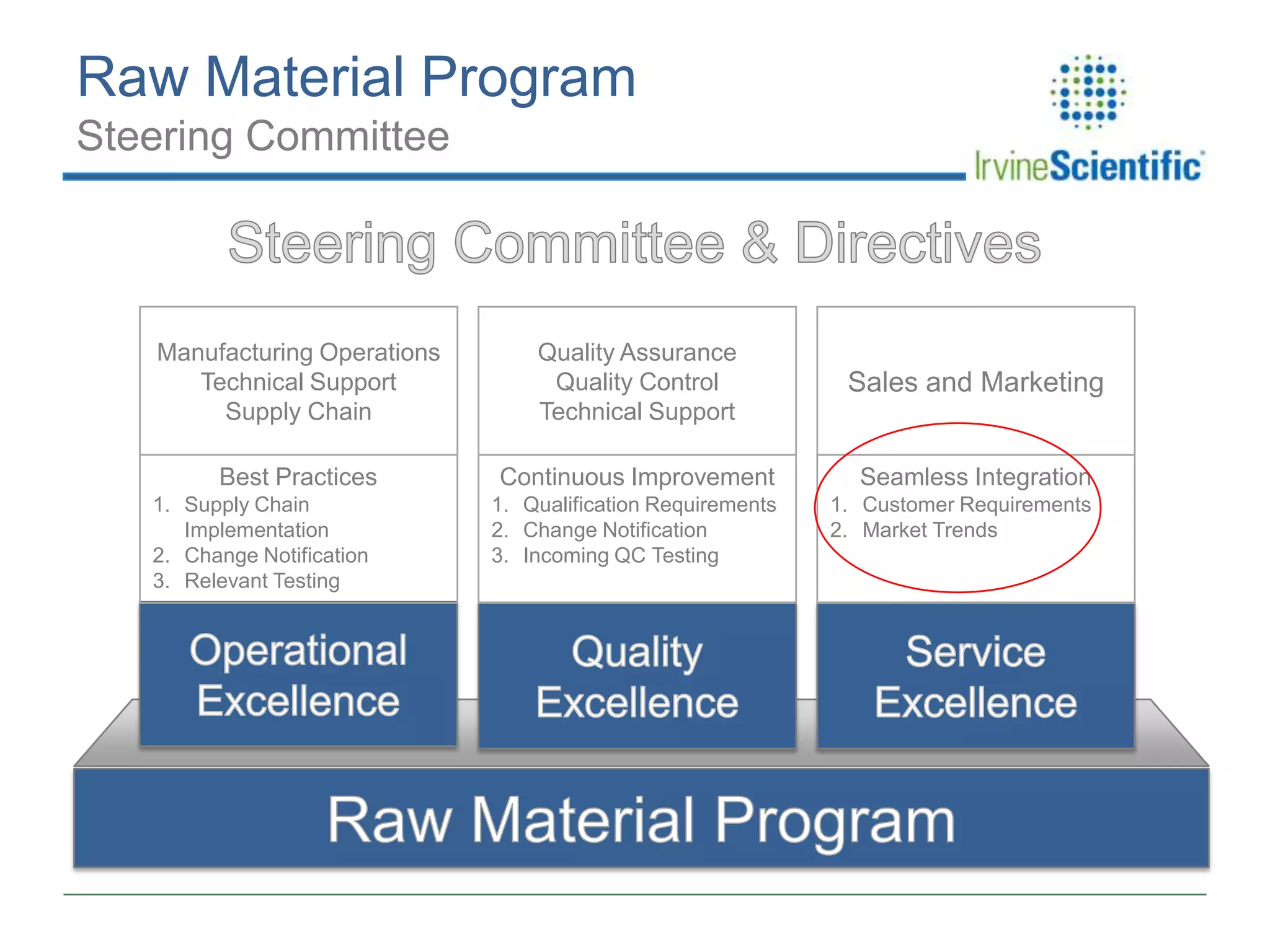
This document summarizes Irvine Scientific's raw material sourcing and management program. It discusses the company's 40-year history in cell culture, the markets it serves, and its parent company. The presentation outlines Irvine's raw material program elements, including historical events of contamination issues, operational excellence through industry best practices like dual sourcing, quality excellence through continuous improvement and analysis, and ensuring service excellence through seamless integration of custom customer needs. It concludes that the program aims to align with industry expectations through a practical risk-based management approach and ensure successful execution through business continuity.