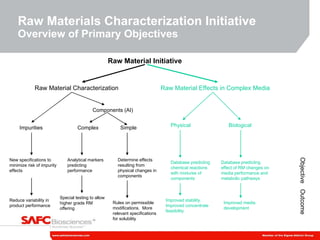
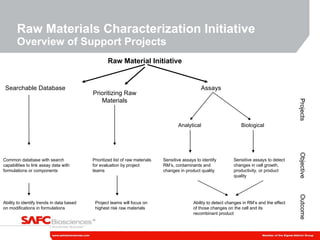
1) SAFC Biosciences is launching a Raw Material Characterization Initiative to reduce variability in media performance and improve response to technical issues by better understanding the impact of raw material changes.
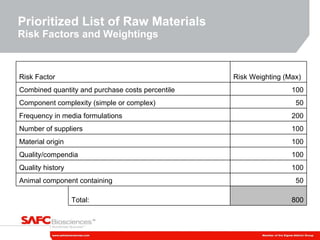
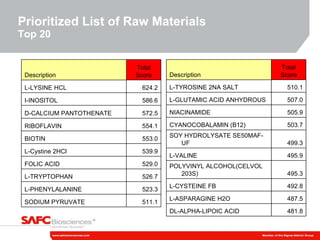
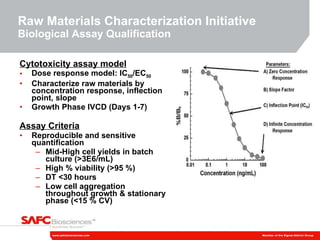
2) The initiative involves characterizing raw material impurities, physical properties, and biological effects to develop assays, databases, and specifications to minimize risks.
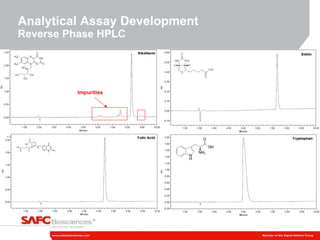
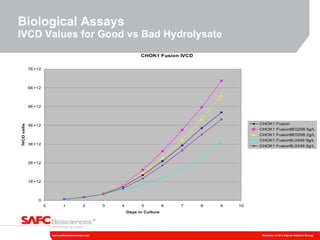
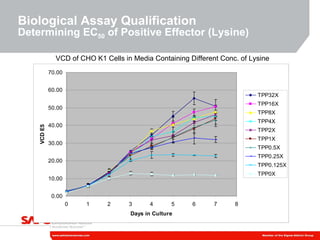
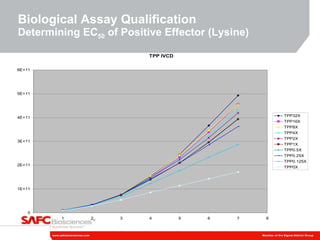
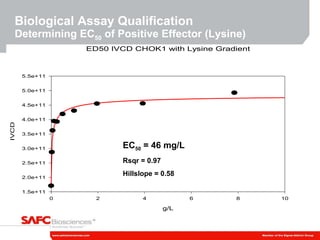
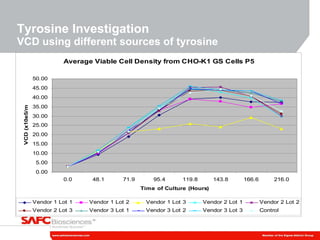
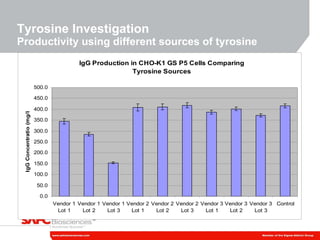
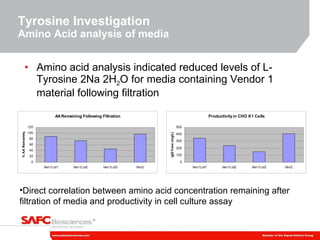
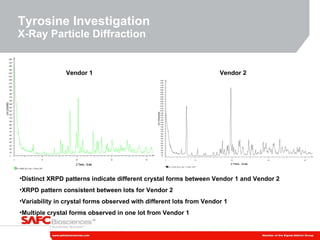
3) As an example, an investigation found that different crystal structures between vendors of L-tyrosine resulted in poor solubility and reduced performance for one vendor, who was then removed.