Report
Share
Recommended
Recommended
More Related Content
What's hot
What's hot (20)
Reactivity order of Pyrrole, Furan and Thiophene.pptx

Reactivity order of Pyrrole, Furan and Thiophene.pptx
Heterocyclic compounds - Furan - Synthesis of furan - Characteristic reaction...

Heterocyclic compounds - Furan - Synthesis of furan - Characteristic reaction...
Similar to THIOPHENE.ppt
Similar to THIOPHENE.ppt (20)
vdocuments.net_heterocyclic-chemistry-58bb82e5b406c.ppt

vdocuments.net_heterocyclic-chemistry-58bb82e5b406c.ppt
Recently uploaded
https://app.box.com/s/7hlvjxjalkrik7fb082xx3jk7xd7liz3TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...

TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...Nguyen Thanh Tu Collection
Recently uploaded (20)
General Principles of Intellectual Property: Concepts of Intellectual Proper...

General Principles of Intellectual Property: Concepts of Intellectual Proper...
This PowerPoint helps students to consider the concept of infinity.

This PowerPoint helps students to consider the concept of infinity.
Presentation by Andreas Schleicher Tackling the School Absenteeism Crisis 30 ...

Presentation by Andreas Schleicher Tackling the School Absenteeism Crisis 30 ...
Russian Escort Service in Delhi 11k Hotel Foreigner Russian Call Girls in Delhi

Russian Escort Service in Delhi 11k Hotel Foreigner Russian Call Girls in Delhi
Unit-V; Pricing (Pharma Marketing Management).pptx

Unit-V; Pricing (Pharma Marketing Management).pptx
Energy Resources. ( B. Pharmacy, 1st Year, Sem-II) Natural Resources

Energy Resources. ( B. Pharmacy, 1st Year, Sem-II) Natural Resources
Measures of Dispersion and Variability: Range, QD, AD and SD

Measures of Dispersion and Variability: Range, QD, AD and SD
TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...

TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...
Measures of Central Tendency: Mean, Median and Mode

Measures of Central Tendency: Mean, Median and Mode
Ecological Succession. ( ECOSYSTEM, B. Pharmacy, 1st Year, Sem-II, Environmen...

Ecological Succession. ( ECOSYSTEM, B. Pharmacy, 1st Year, Sem-II, Environmen...
THIOPHENE.ppt
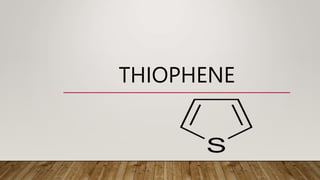
- 1. THIOPHENE
- 2. INTRODUCTION • Thiophene is a heterocyclic compound with the formula C4H4S • It consists of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions • Thiophene is aromatic because it has six π electrons in a planar, cyclic, conjugated system • It occurs in the light oil fraction of coat-tar and is usually present as an impurity in commercial benzene.
- 3. PREPARATION METHODS • (1) By passing a mixture of acetylene and hydrogen sulphide through a tube containing aluminium oxide at 400°C.
- 4. (2) BY HEATING SODIUM SUCCINATE WITH PHOSPHOROUS TRISULPLSIDE
- 5. • 3. By the high-temperature (650 C) reaction of sulphur with butane. (Commercial Method of Preparation).
- 6. FROM COAL TAR BENZENE • The boiling points of thiophene and benzene are so close together that they cannot be separated by distillation. • Thiophene may be separated from benzene by shaking the mixture with cold concentrated sulphuric acid which sulphonates it more readily than benzene • Thiophene sulphonic acid is then removed from the solution by the usual methods and converted into thiophene by super-heated steam • Best method of removing thiophene from benzene is by shaking with Raney nickel.
- 7. • A simple method is to boil benzene-thiophene mixture with mercuric acetate. • Thiophene s converted into the insoluble 1-merouriacetate which regenerates thiophene on boiling with concentrated hydrochloric acid.
- 8. PHYSICAL PROPERTIES • Thiophene is a colourless liquid, boiling point 84 C, with an odour very similar to that of benzene • It is insoluble in water, but miscible with most organic solvents • It is comparatively stable to oxidation • Thiophene also has alternate double and single bond structure and the aromatic character in it is very pronounced as compared to furan and pyrrole.
- 9. CHEMICAL PROPERTIES • Thiophene is 300 times more reactive than benzene • Thiophene does not show any basic properties • It is much more stable to acids than either pyrrole or furan • Thiophene does not undergo the Diels-Alder reaction unlike pyrrole or furan
- 10. 1) ELECTROPHILIC SUBSTITUTIONS: • Thiophene, like furan and pyrrole, undergoes electrophilic substitution reactions primarily at C-2. Substitution at C-3 occurs only when both of the 2-positions (α and ά) are already occupied.
- 12. (2) REDUCTION: • Thiophene may be hydrogenated by means of sodium amalgam and ethanol to tetrahydrothiophene.
- 13. (3) DESULPHURISATION: • Catalytic reduction of Thiophene with Raney Nickel results in the removal of Sulphur to form n-butane
- 14. PHARMACEUTICAL IMPORTANCE OF THIOPHENE • In medicine, thiophene derivatives shows antimicrobial, analgesic and anti- inflammatory, antihypertensive, and antitumor activity • It is commonly used as a building block in drugs • It is considered to be a structural alert, as its metabolism can lead to the formation of reactive metabolites • Several commercially available drugs such as Tipepidine, Timepidium Bromide, Dorzolamide, Tioconazole, Citizolam, Sertaconazole Nitrate contain thiophene nucleus