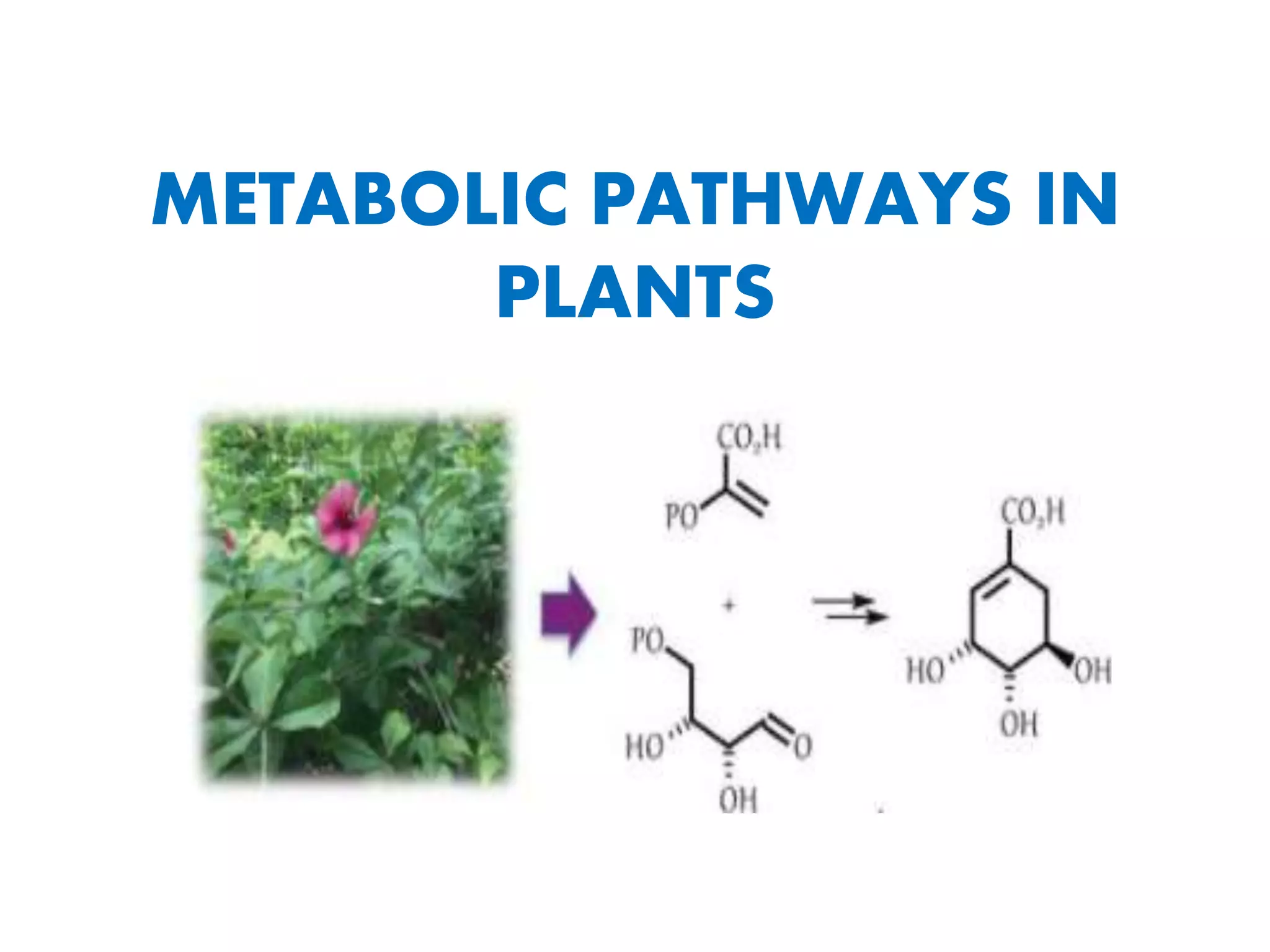
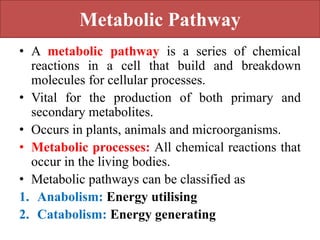
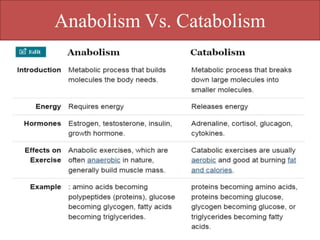
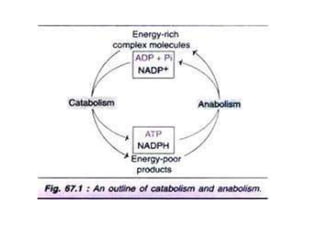
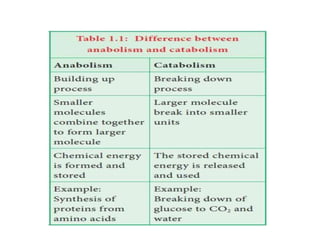
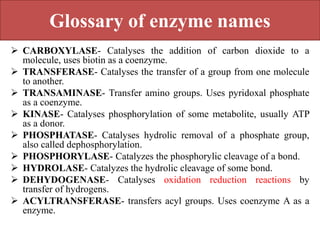
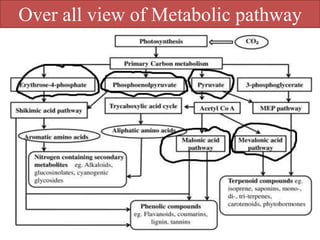
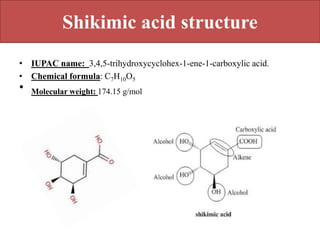
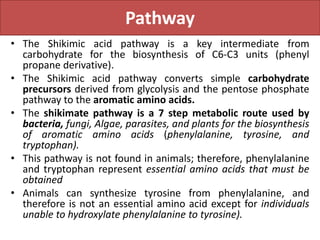
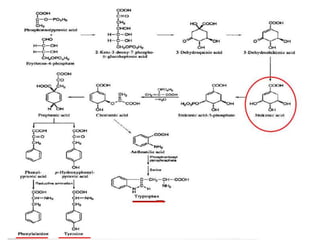
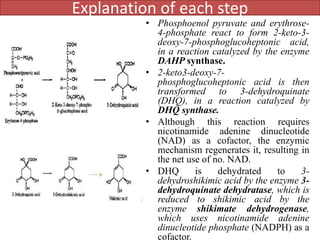
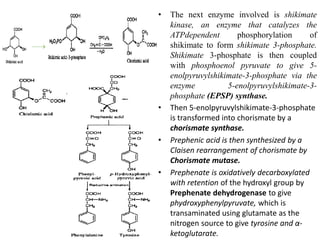
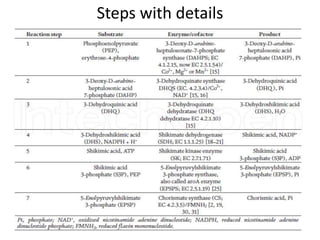
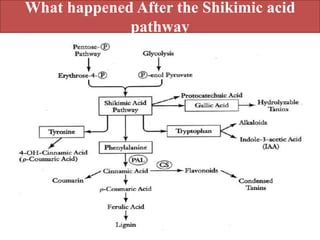
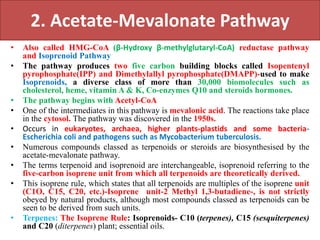
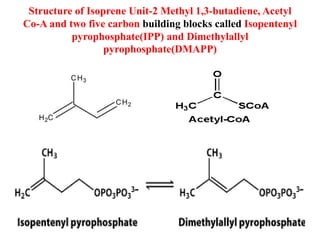
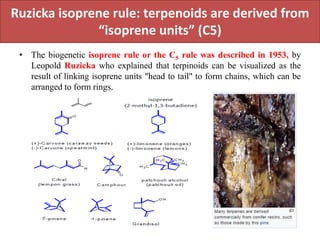
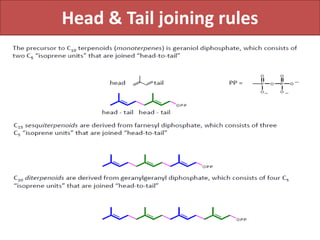
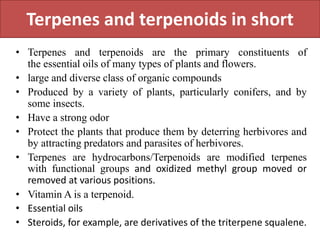
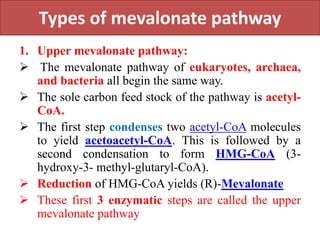
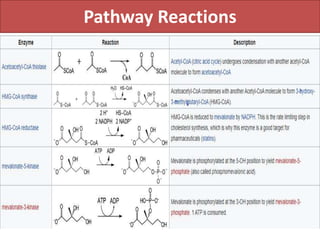
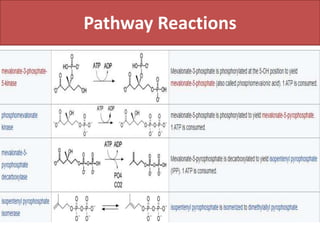
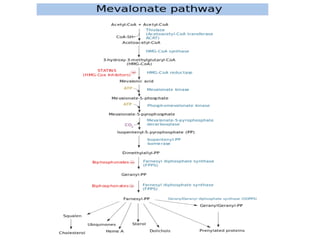
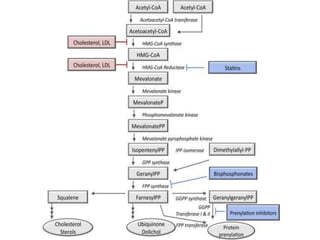
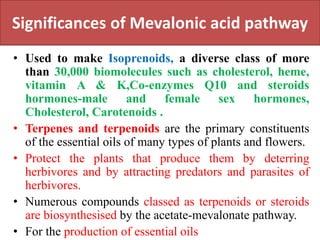
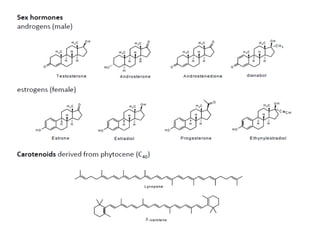
Here are the key points about amino acid pathways:
- Essential amino acids: phenylalanine, valine, threonine, tryptophan, methionine, leucine, isoleucine, lysine, histidine, arginine
- Non-essential amino acids: alanine, asparagine, aspartate, cysteine, glutamate, glutamine, glycine, proline, serine, tyrosine
Biosynthesis pathways:
- Glutamate: Derived from α-ketoglutarate in the Krebs cycle. Reduced to glutamate by NADPH-dependent glutamate dehydrogenase.
- Histidine: Synthesized from phosph