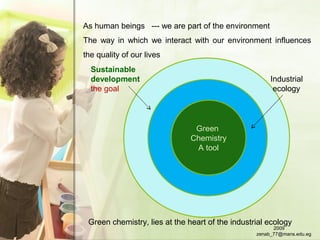
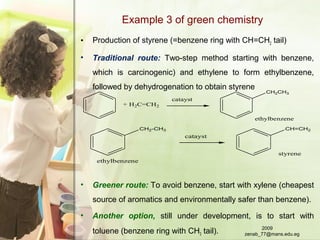
The document discusses green chemistry and its importance for sustainability. It defines green chemistry as the design of chemical products and processes to reduce use and generation of hazardous substances. The principles of green chemistry are described, including preventing waste, safer chemicals and products, and renewable feedstocks. Examples are given of more environmentally friendly routes for chemical reactions compared to traditional methods. The document emphasizes that green chemistry education is key to promoting more sustainable development. In conclusion, it is noted that green chemistry aims to prevent pollution and sustain the Earth.