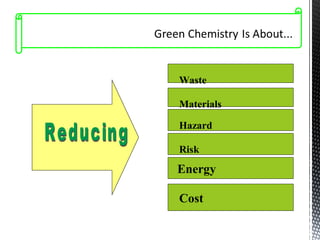
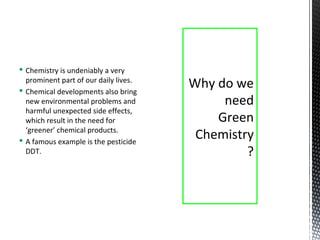
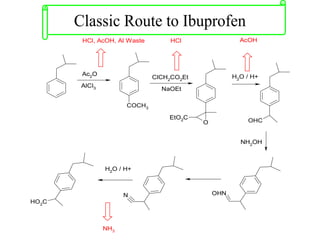
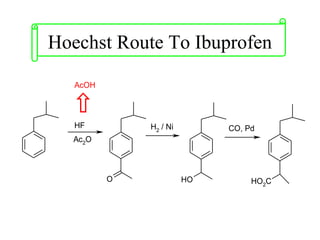
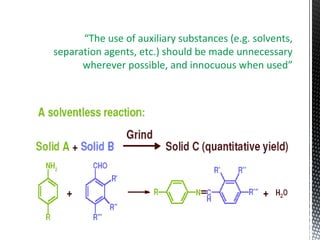
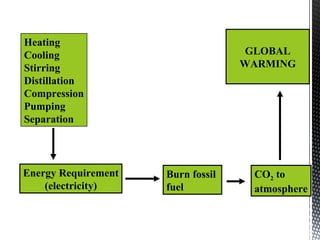
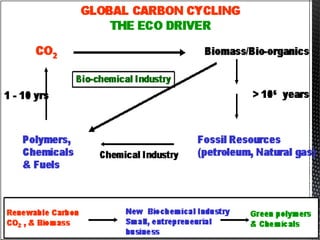
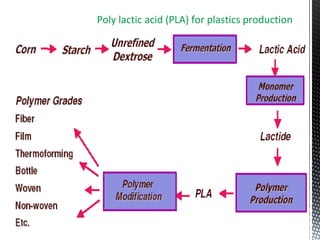
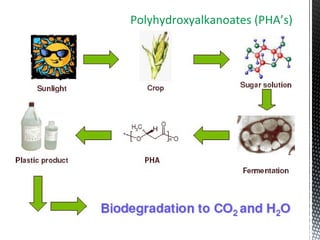
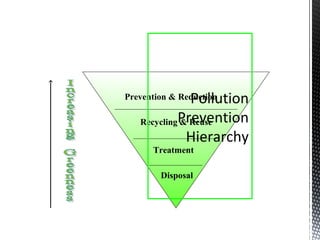
Green chemistry focuses on reducing or eliminating hazardous substances in chemical processes by employing principles such as waste minimization, the use of non-toxic reagents, and renewable resources. It emphasizes pollution prevention at the molecular level and supports environmentally friendly chemical processes that align with its twelve principles. The document discusses the significance of green chemistry in addressing environmental issues, resource depletion, energy efficiency, and sustainable food production.