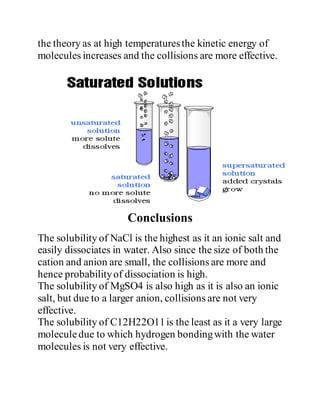
This document describes an experiment to measure the solubility of sodium chloride (NaCl), magnesium sulfate (MgSO4), and sucrose (C12H22O11) in water. The experimental procedure involves making saturated solutions of each solute in water and measuring how much can be dissolved. Observations show that solubility increases with temperature for all substances. NaCl has the highest solubility due to its small ion size, while sucrose has the lowest solubility due to its large molecular size. The results agree with theories about how solubility is affected by solute-solvent interactions and temperature.