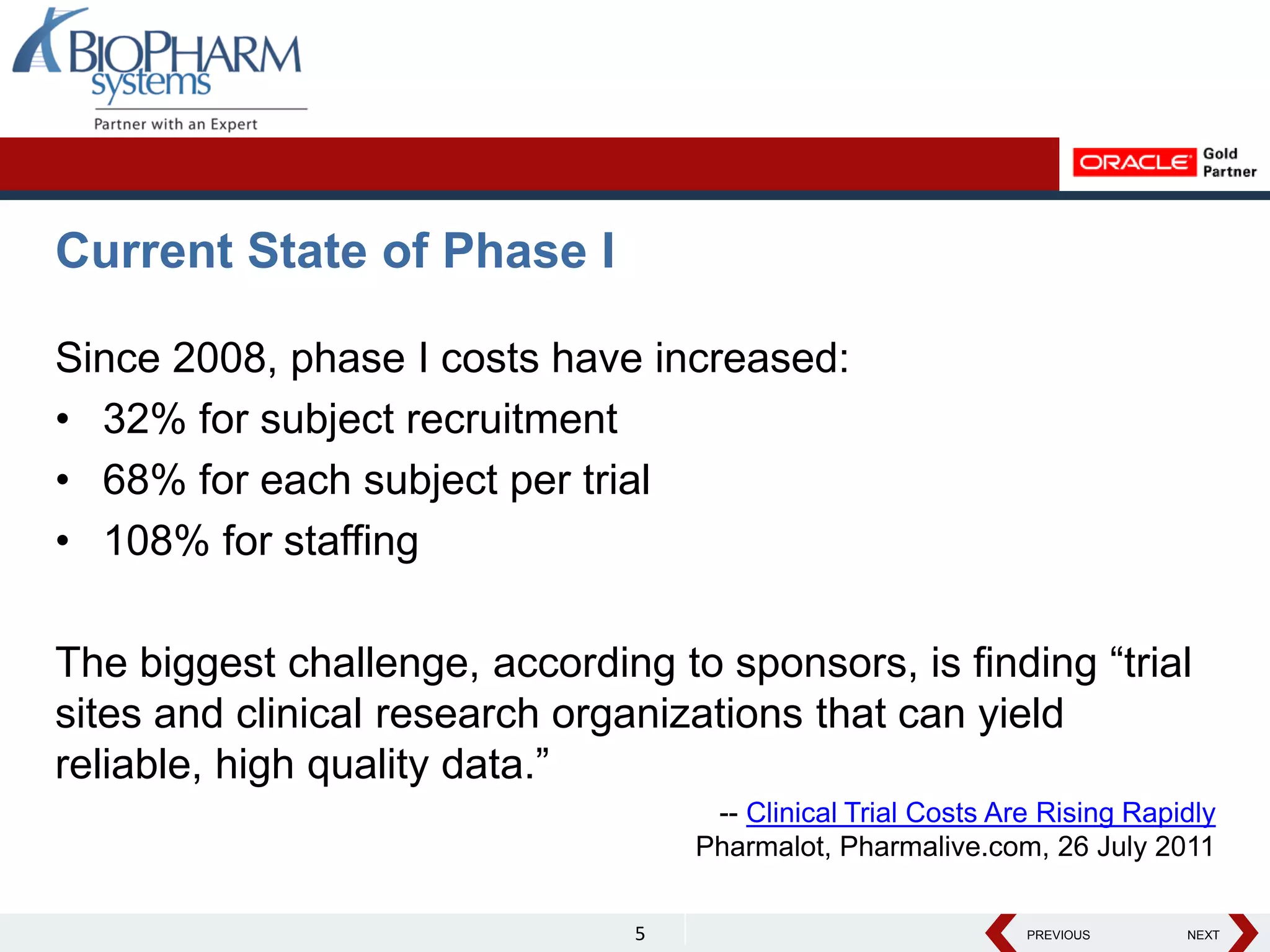
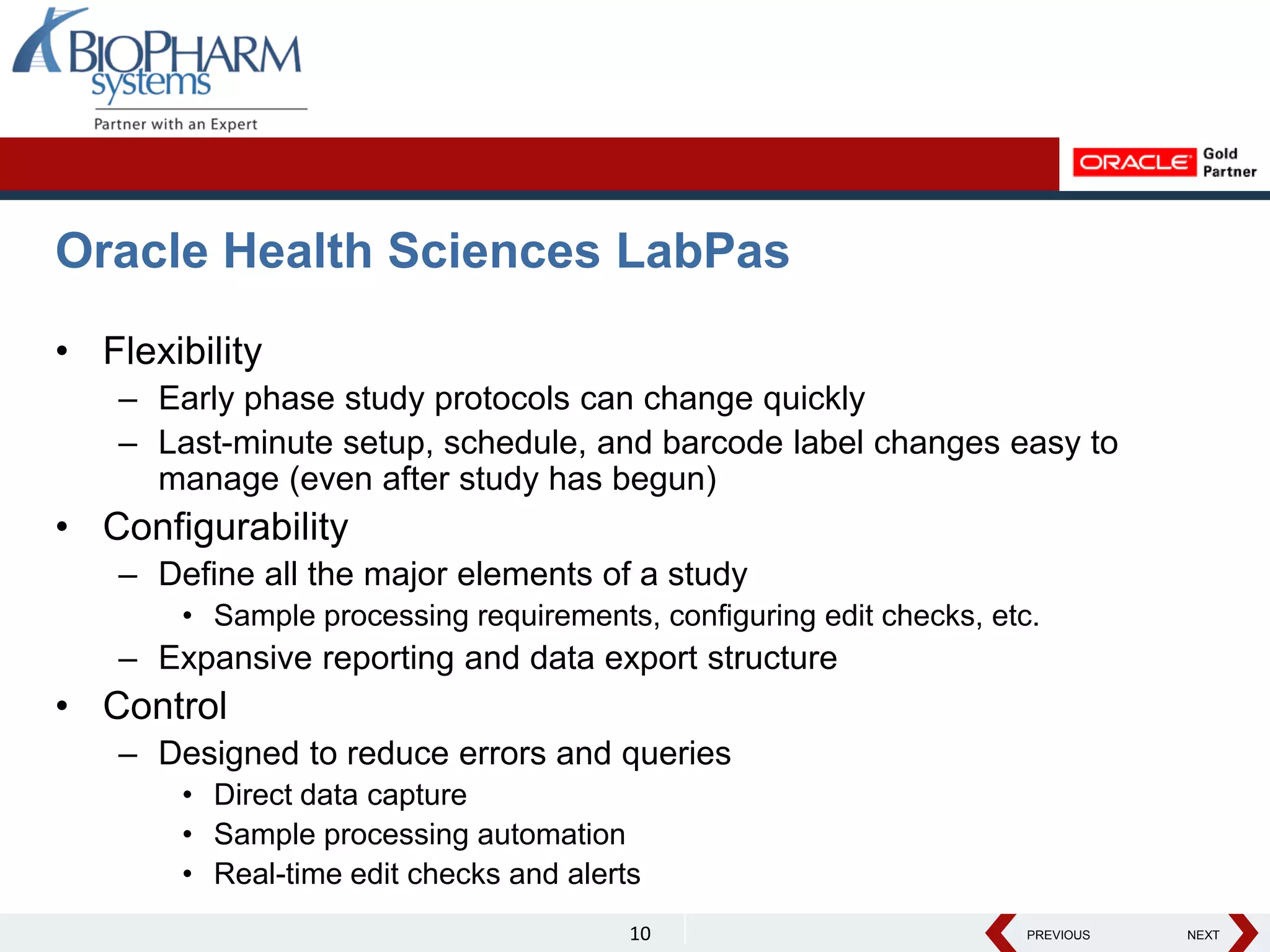
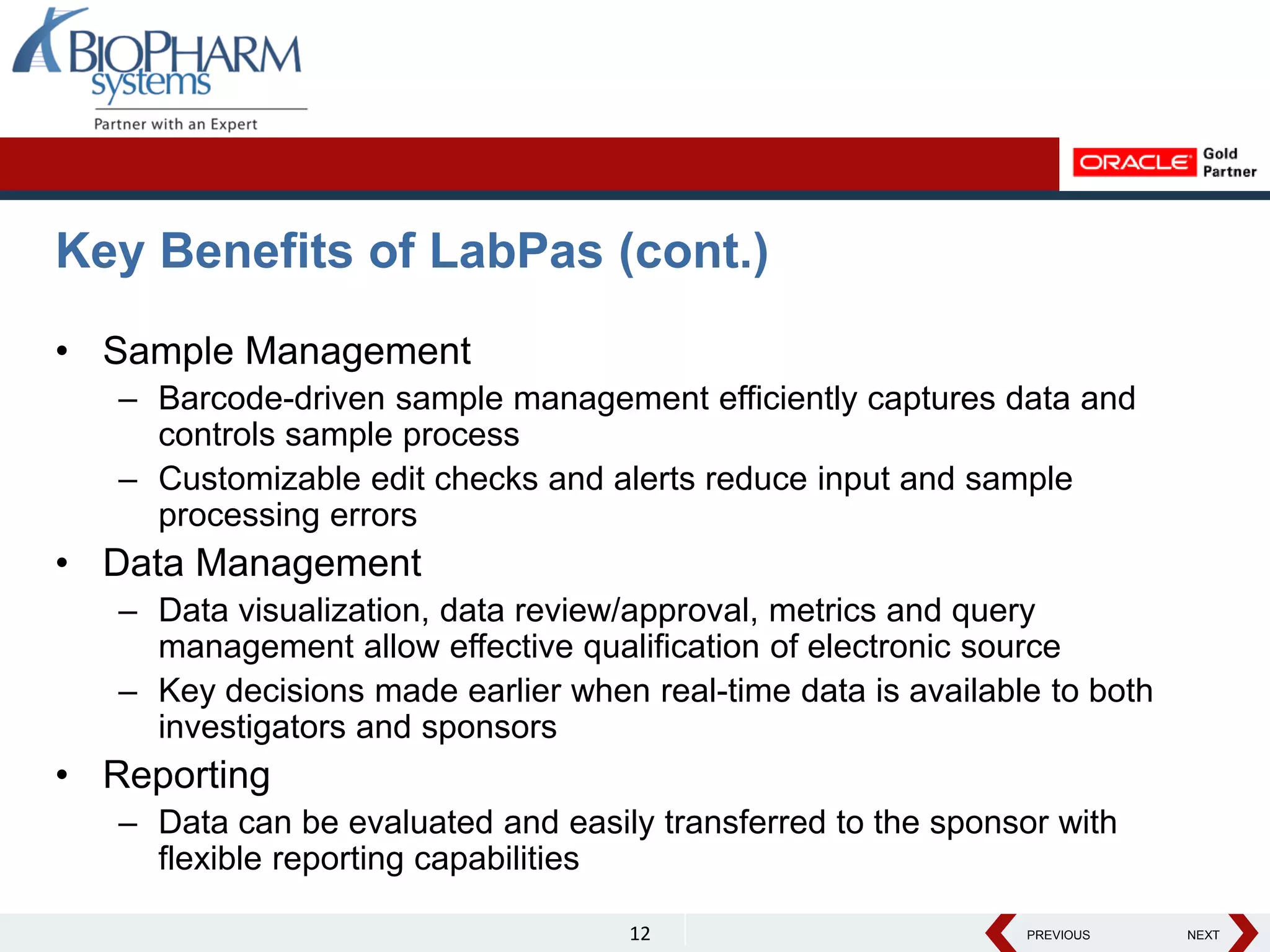
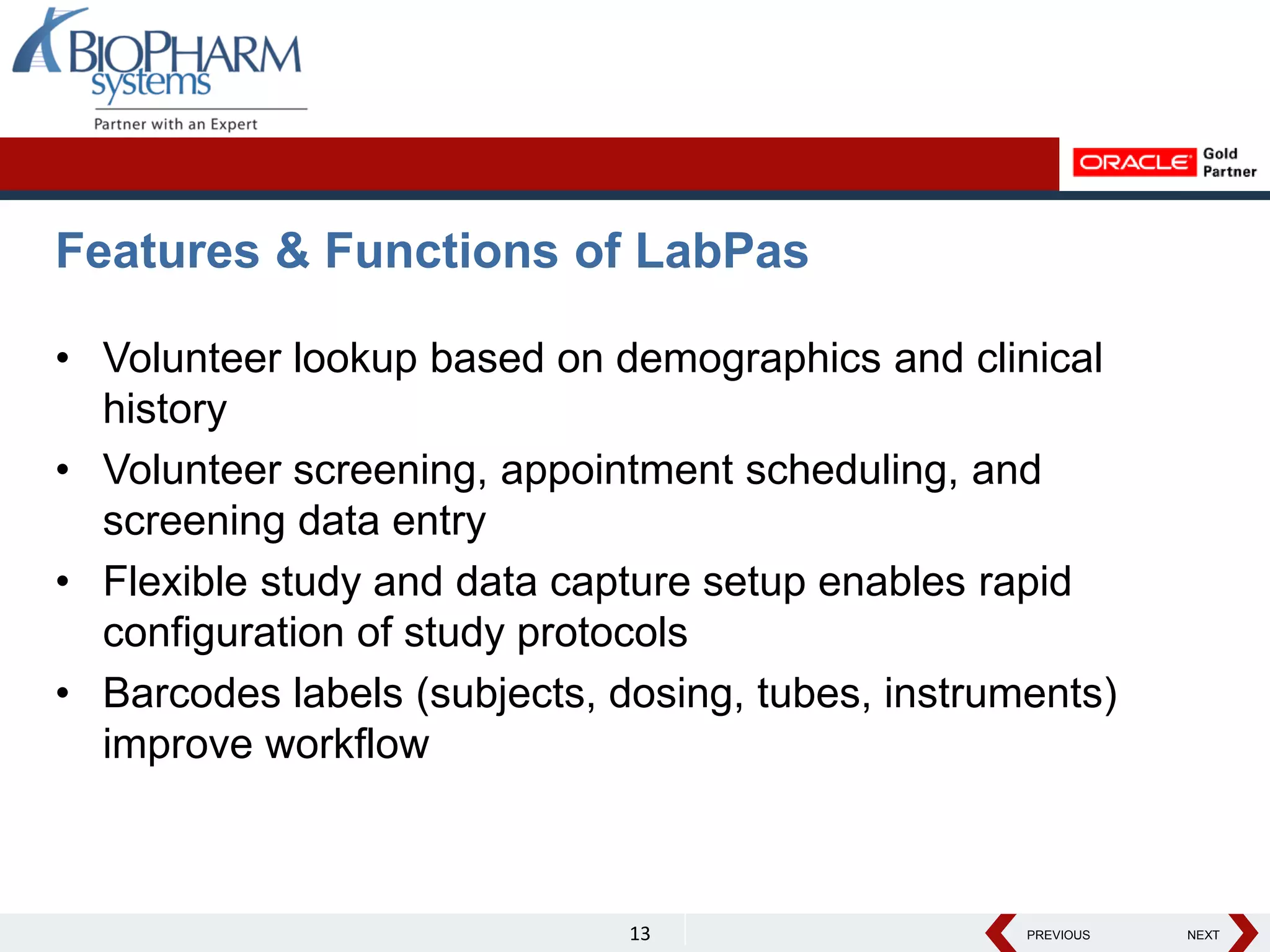
The document discusses the trend toward automating phase I clinical trials. Historically, phase I trials have been largely paper-based, which has led to challenges around volunteer recruitment, complex workflows, scheduling studies, ensuring sample integrity, and accurately capturing data. However, there is a growing trend toward automating phase I trials using solutions like Oracle's LabPas in order to address these challenges, improve efficiency, and help phase I clinics and sponsors better manage costs, quality, and speed of data delivery. The document outlines key benefits and features of LabPas for automating phase I trials.