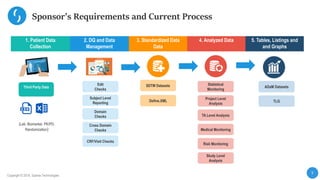
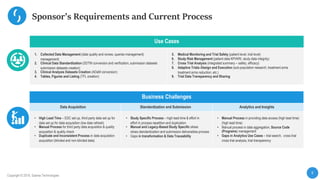
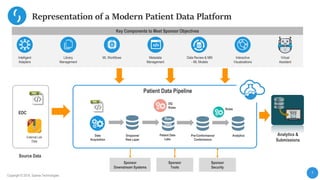
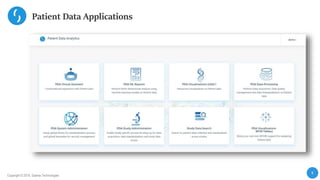
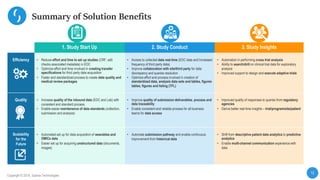
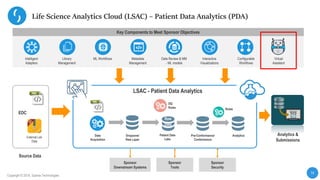
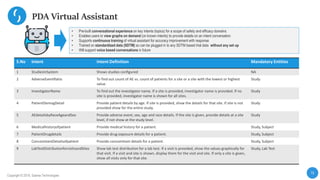
This document describes a next-generation clinical/scientific data management solution presented by Saama Technologies. It discusses the components and benefits of building a patient data analytics solution, including reducing clinical trial costs and timelines through improved data acquisition, standardization, and analytics. The solution aims to address current challenges around clinical data management by providing a modern patient data platform with features like a patient data lake, metadata management, and machine learning capabilities.