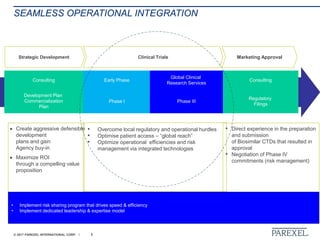
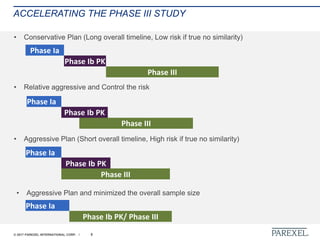
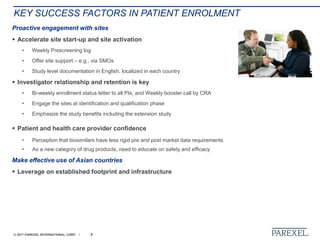
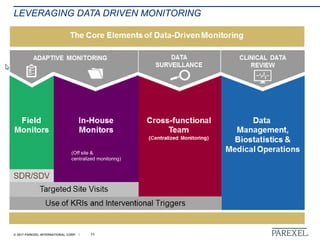
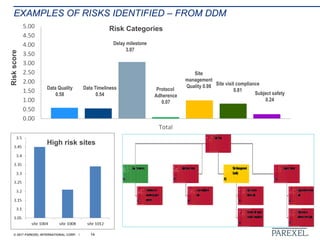
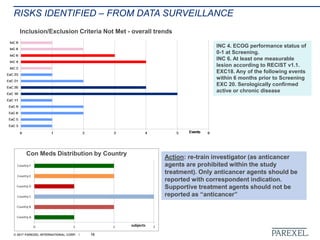
The document discusses effective pathways for late-stage biosimilar clinical trials. It outlines strategies for optimizing patient recruitment and retention, leveraging data-driven monitoring, and enhancing operational efficiencies. Specific considerations include understanding regulatory environments, selecting appropriate reference products and clinical endpoints, identifying qualified investigator sites, and employing risk-based monitoring approaches using data surveillance technologies. The presentation emphasizes that innovative trial designs and leveraging data-driven approaches will be important to efficiently reach biosimilar approval in the intensifying race to launch new products.