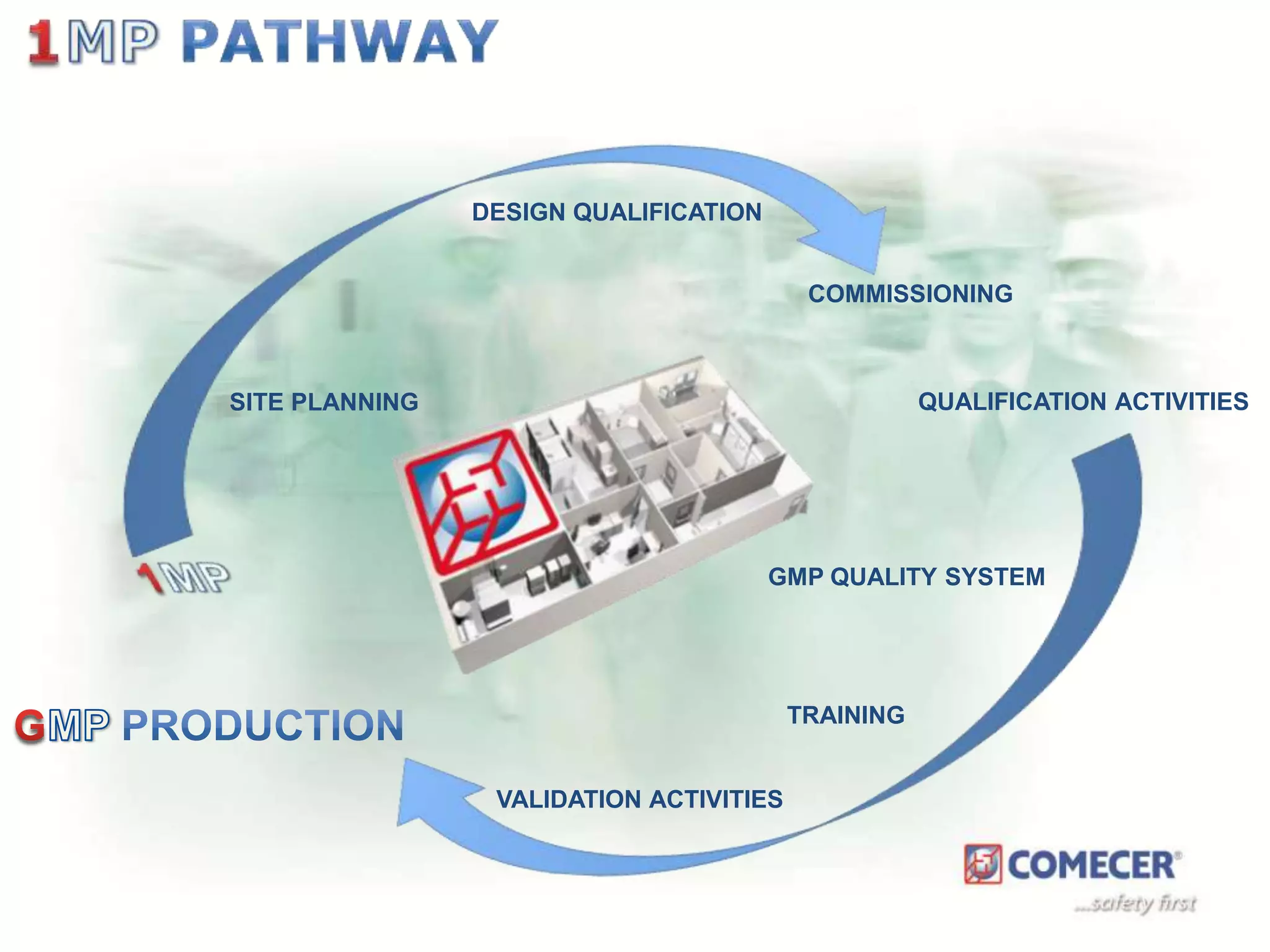
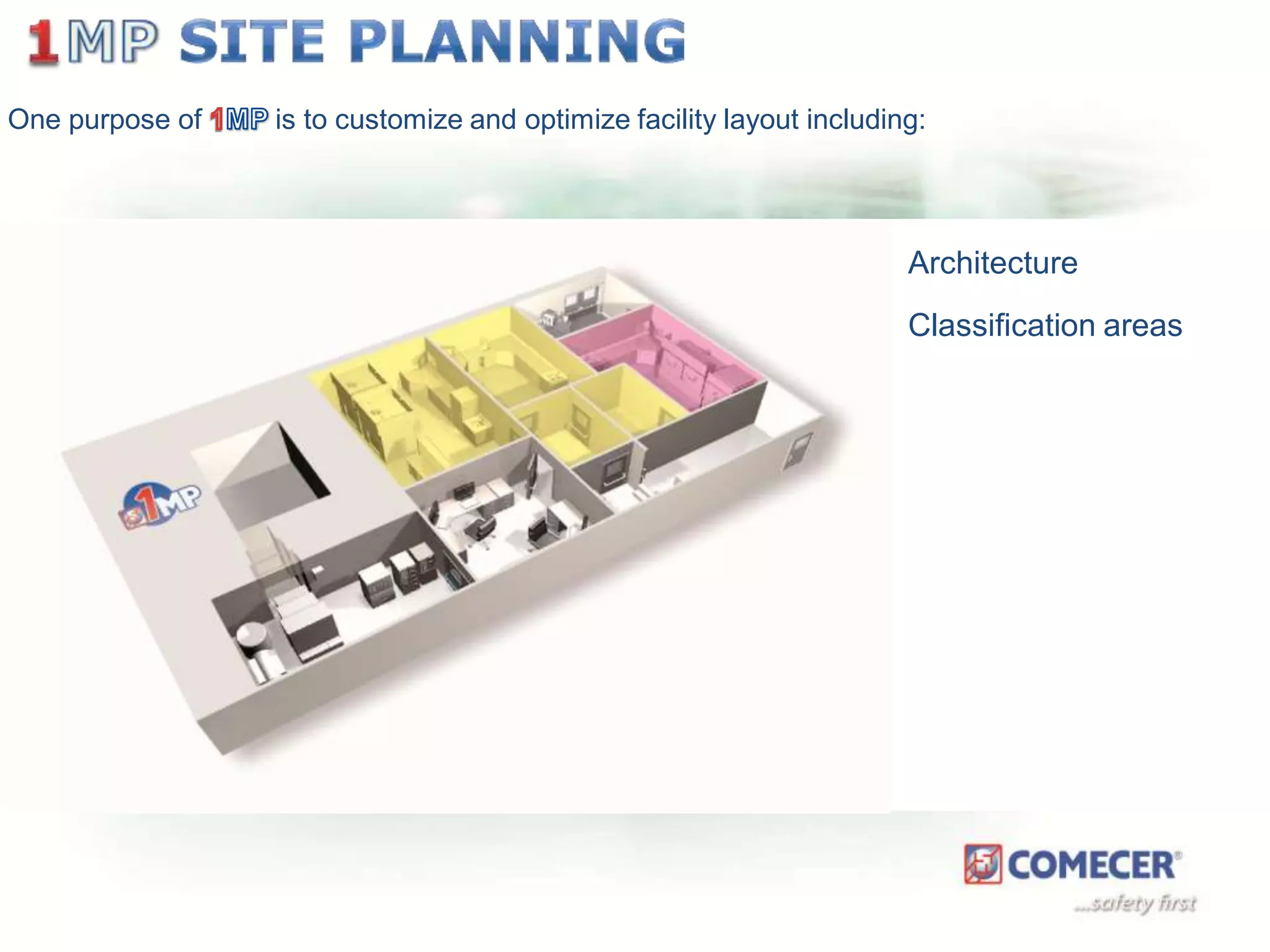
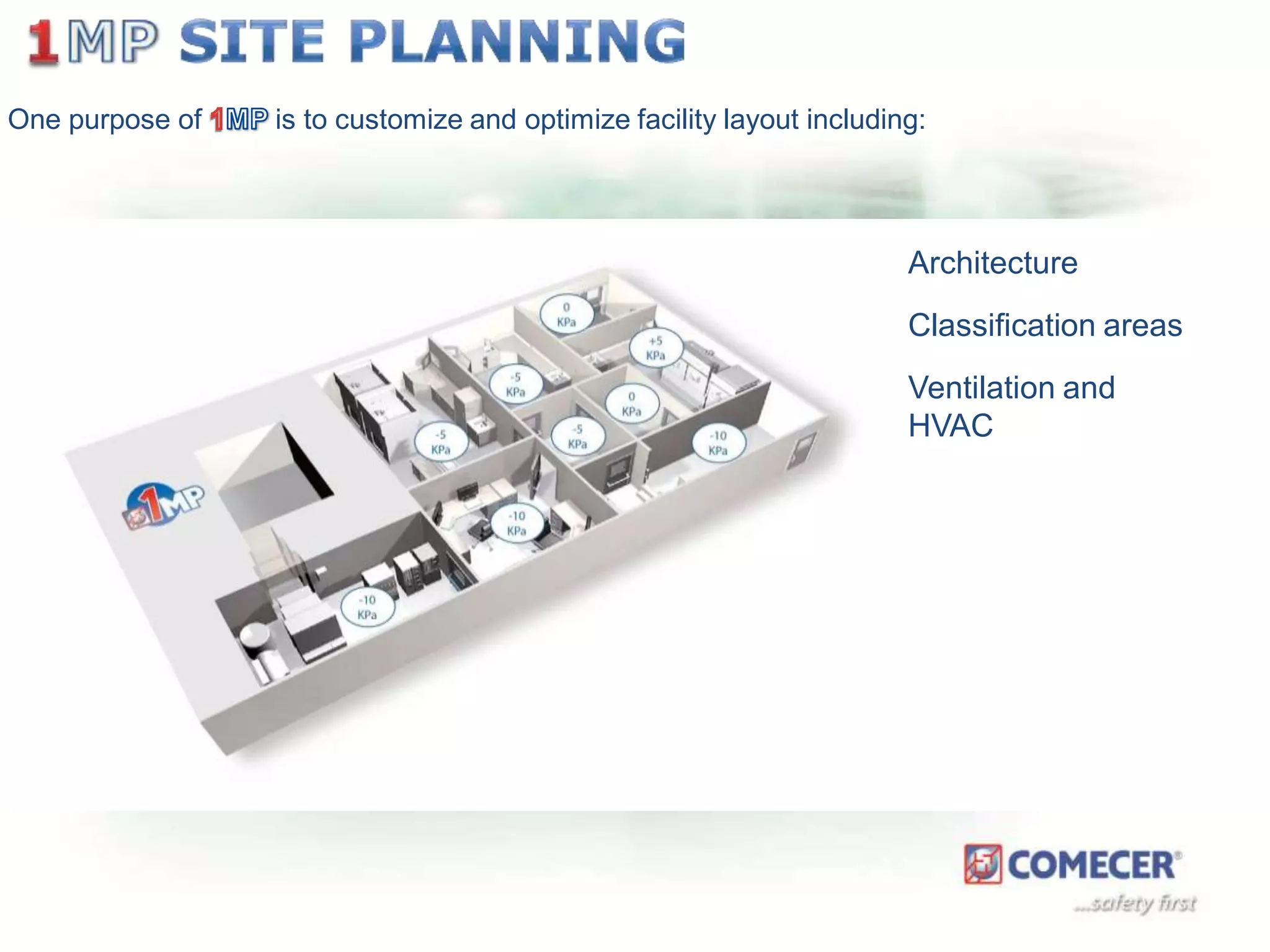
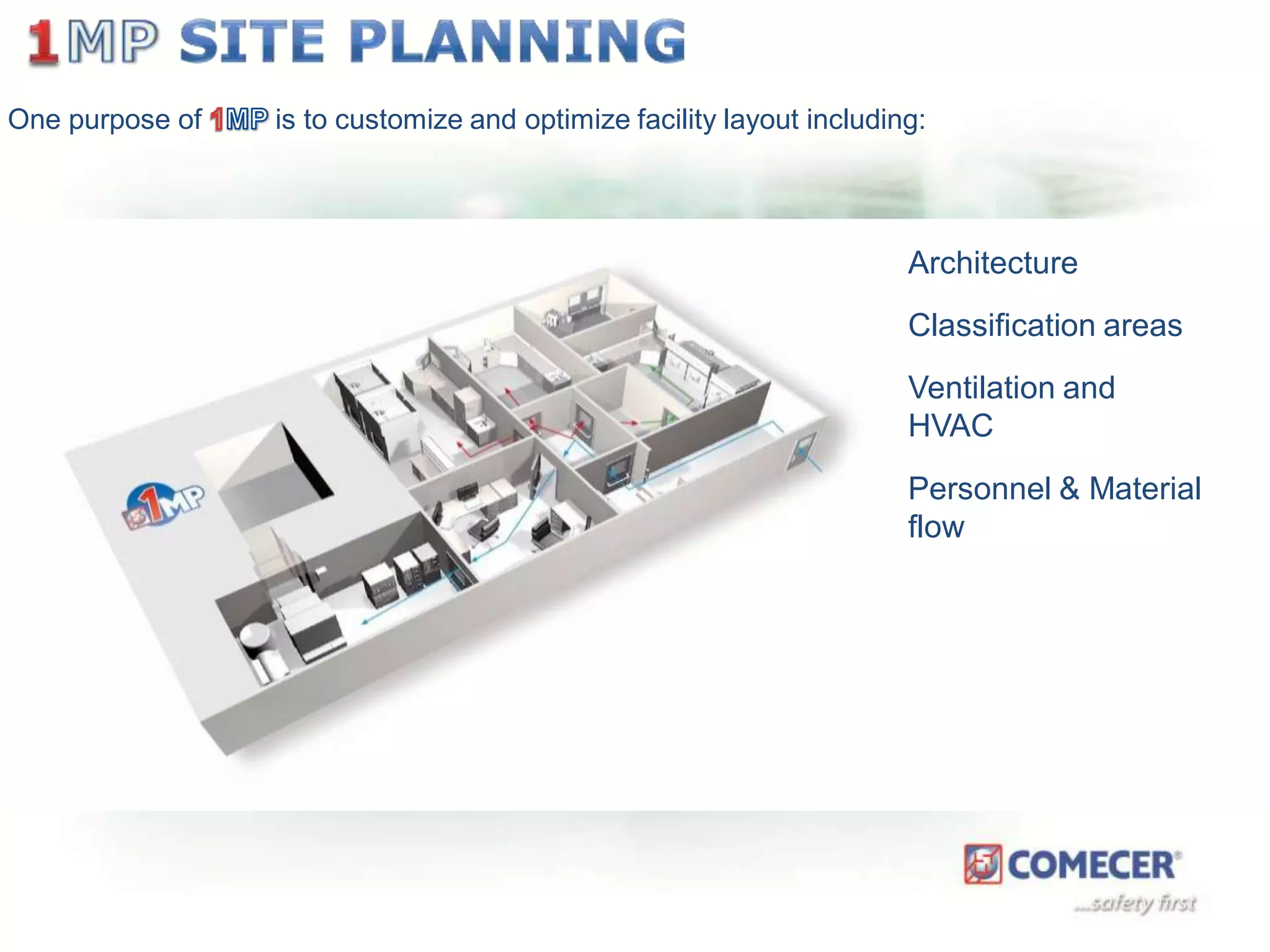
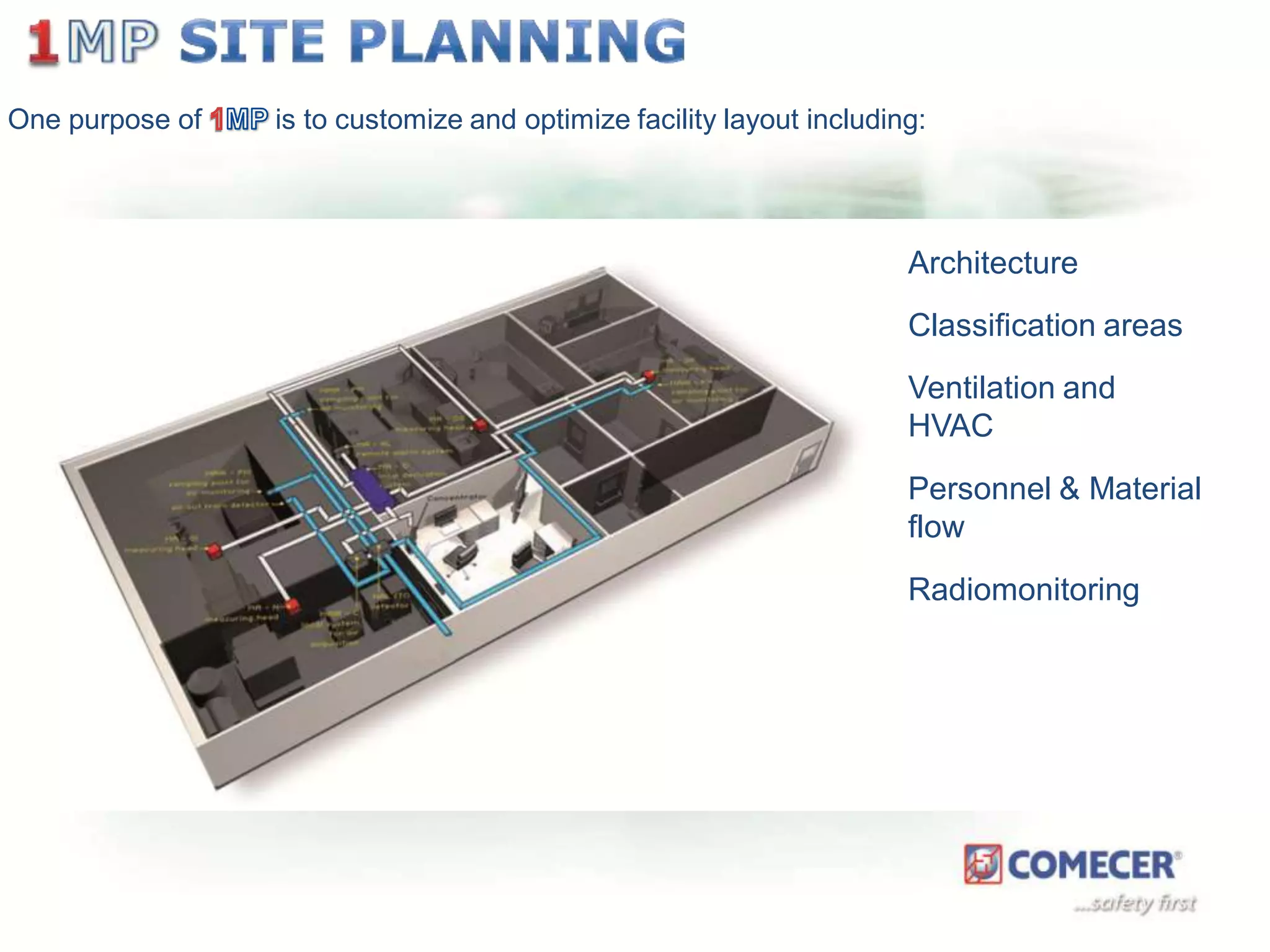
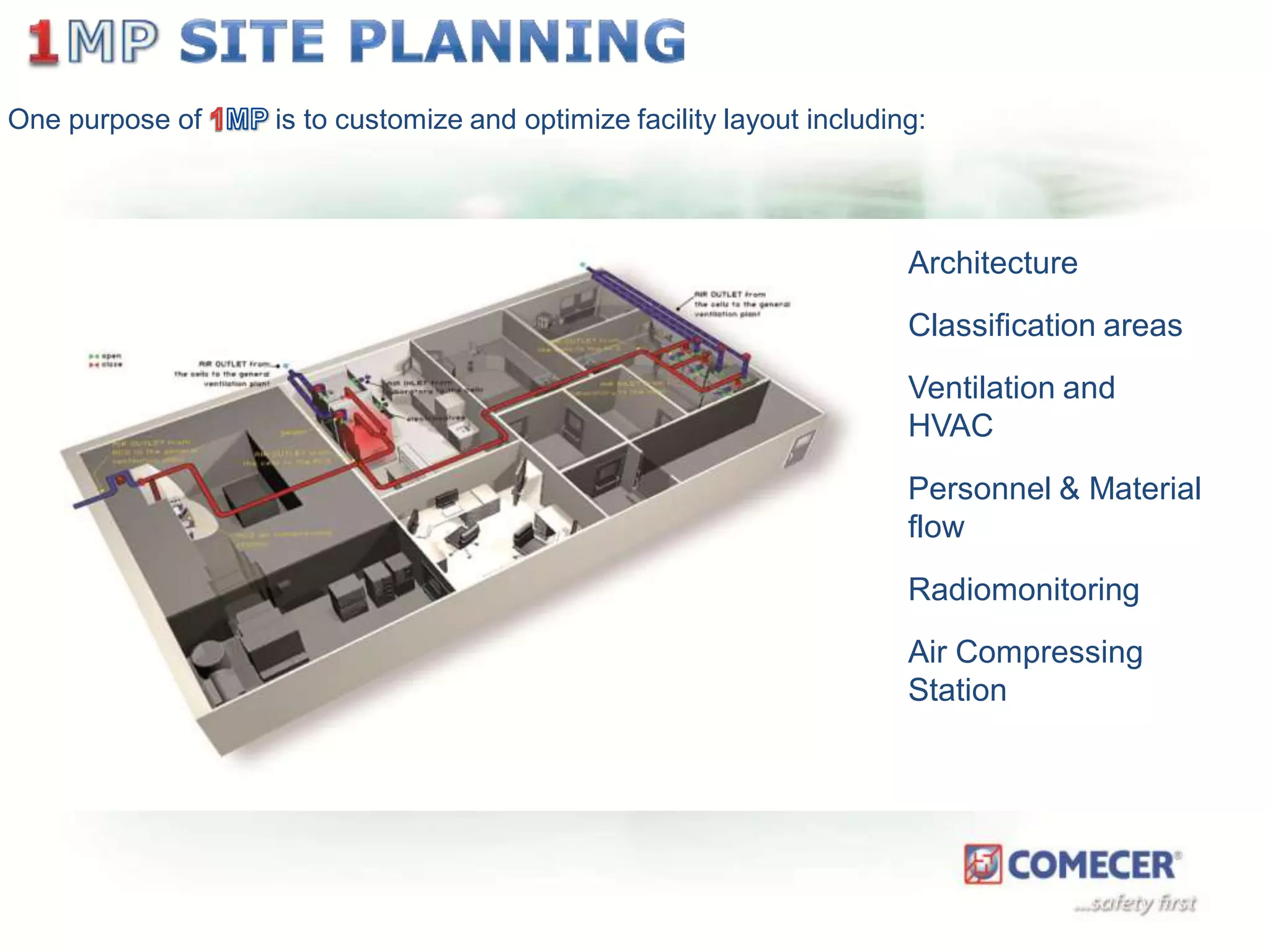
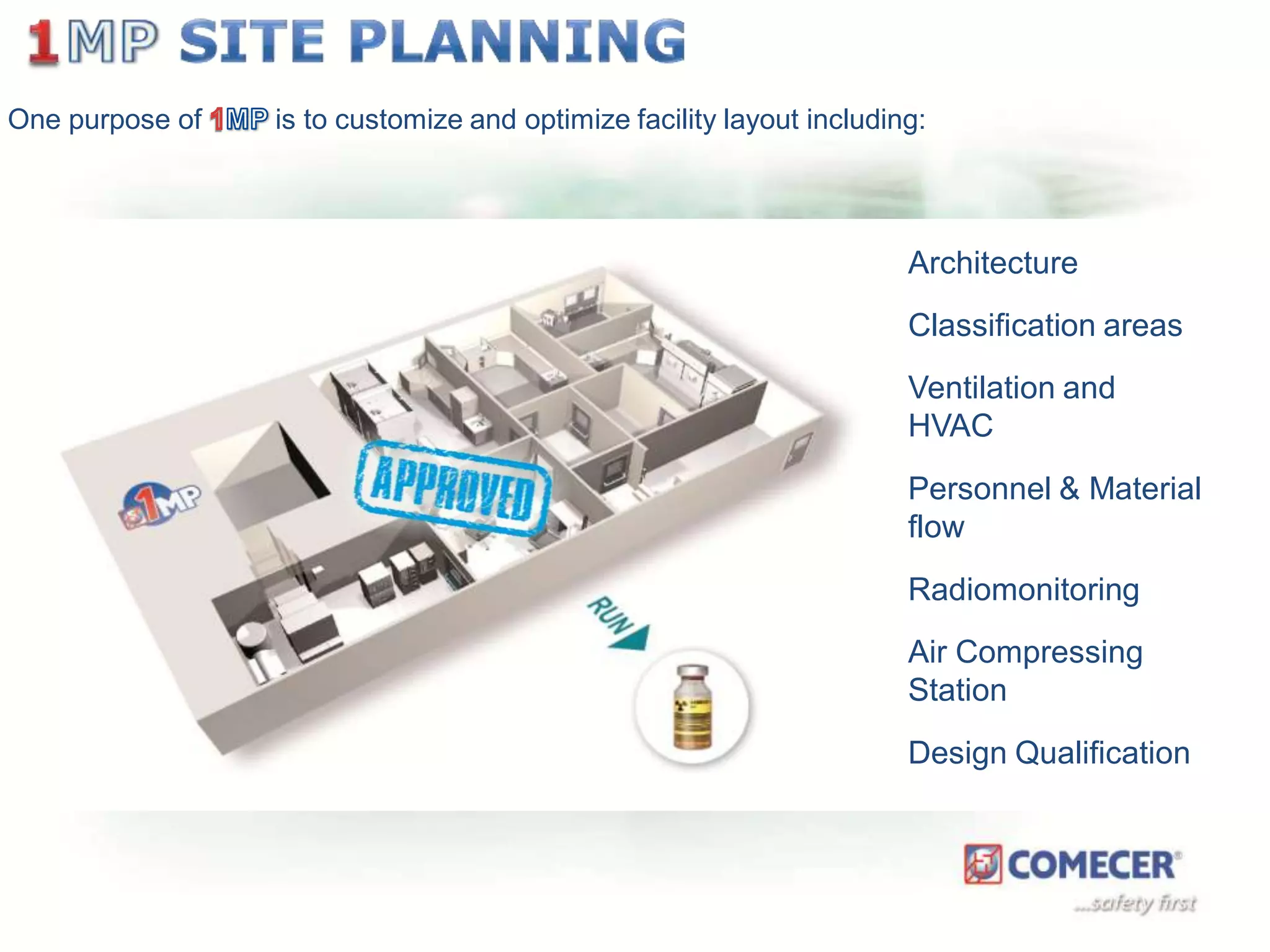
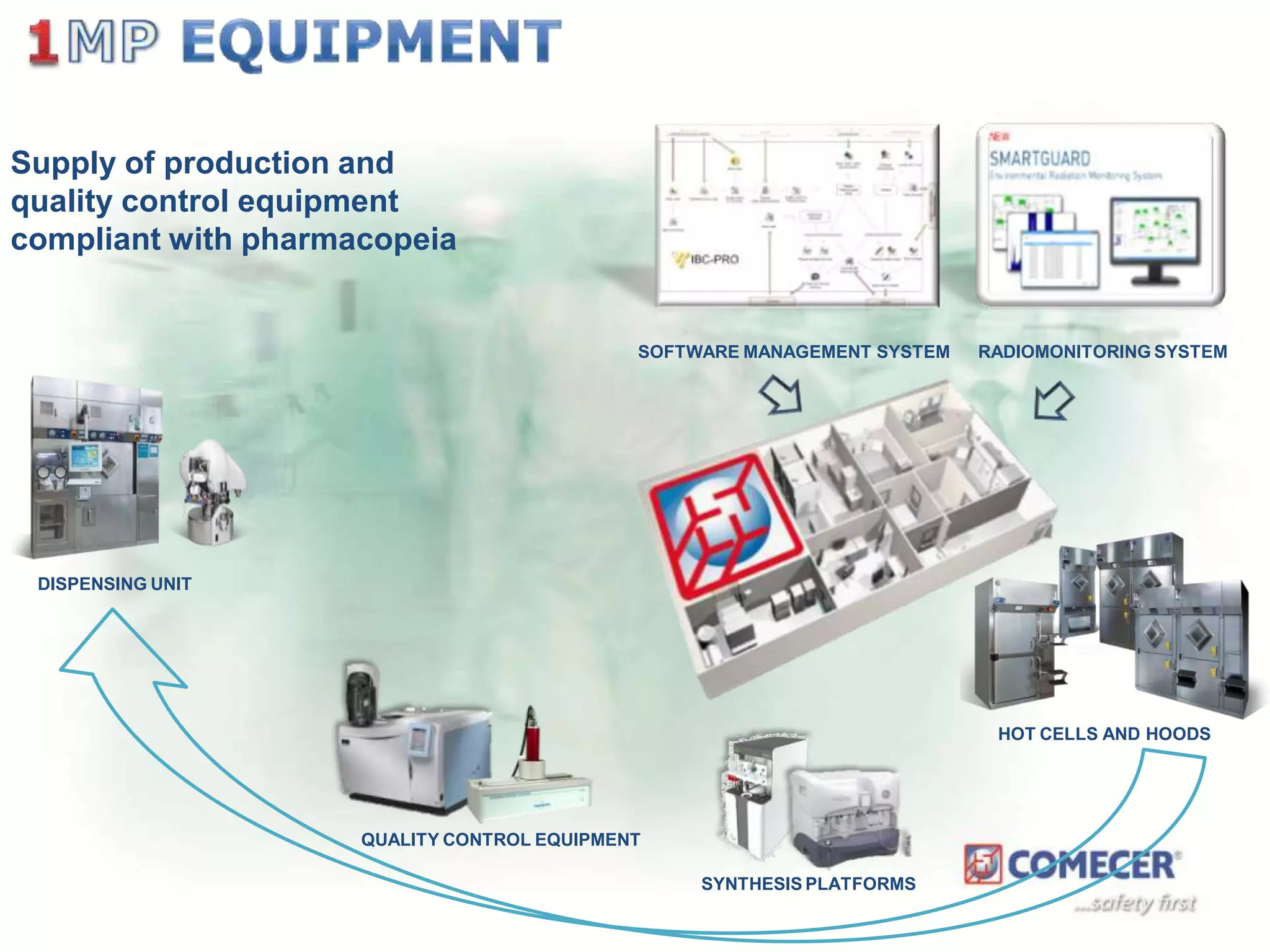
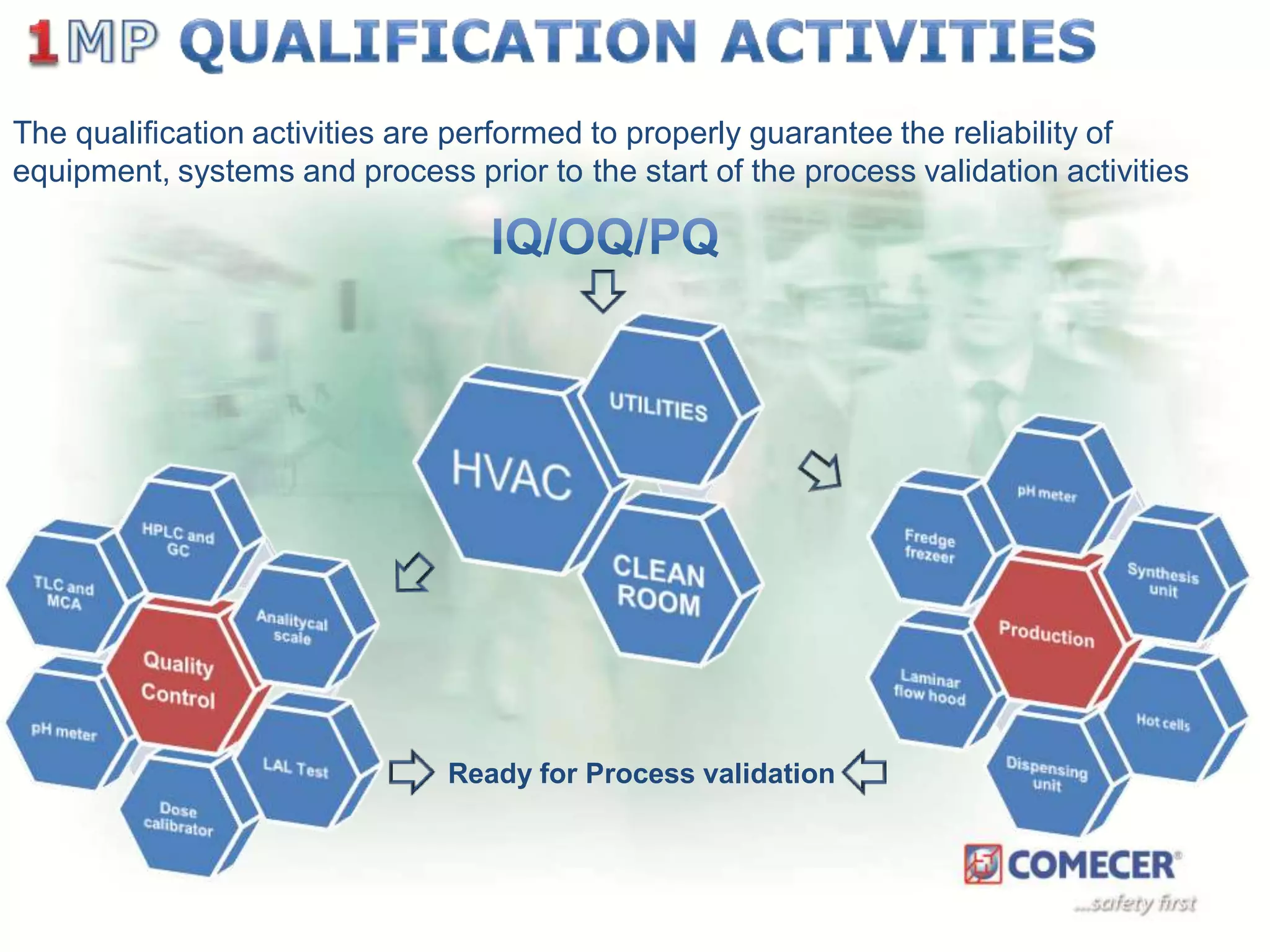
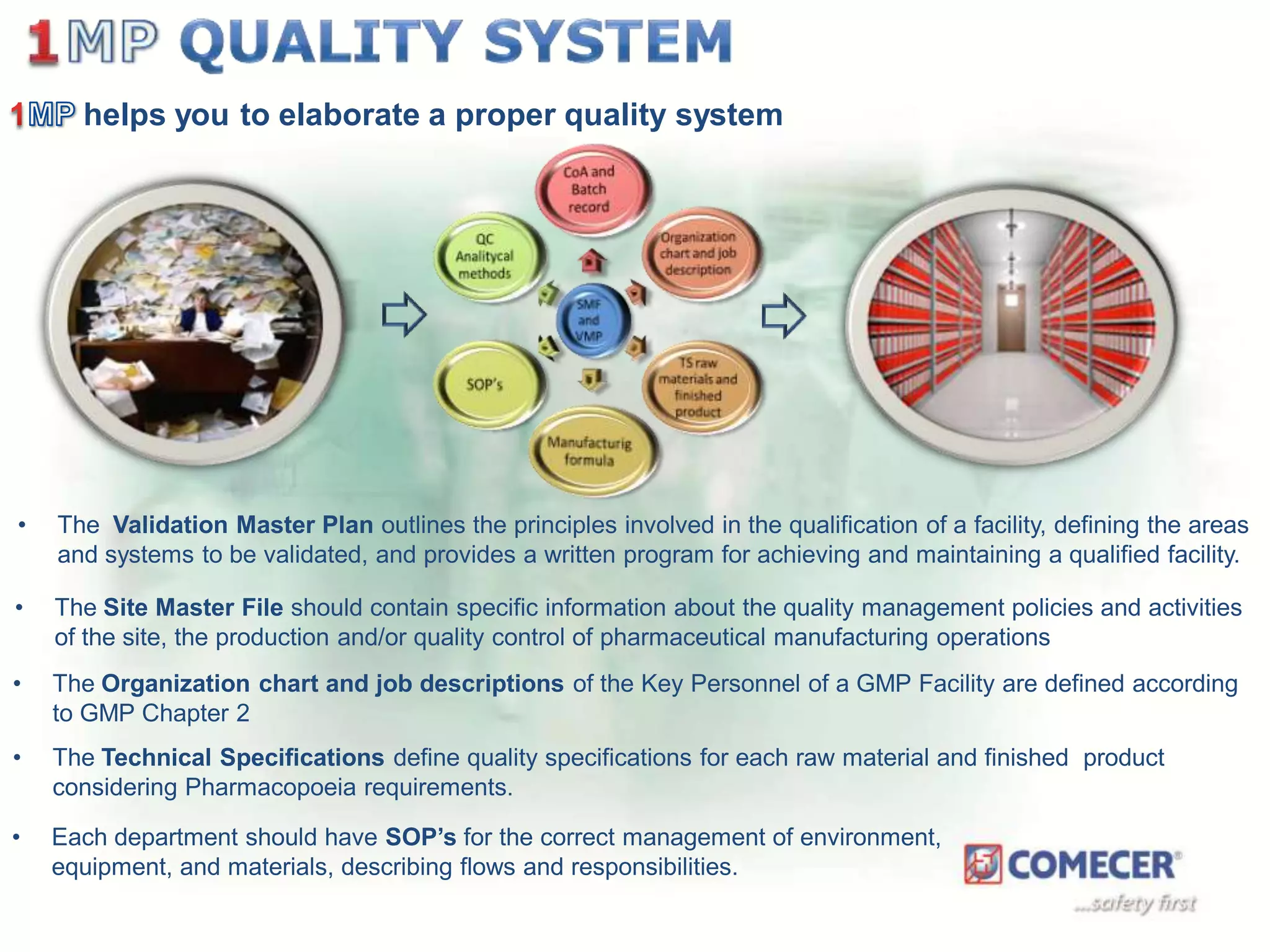
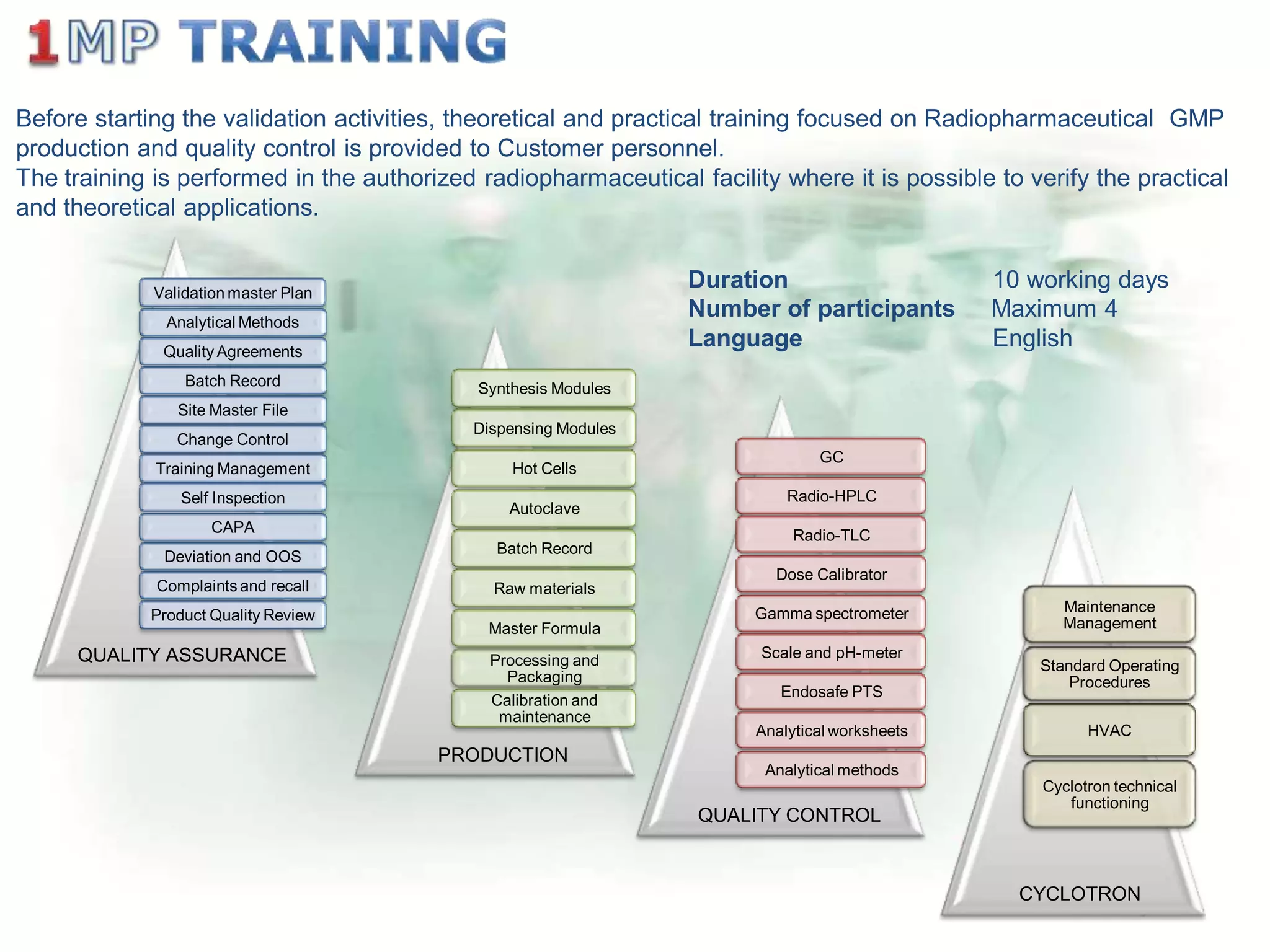
The document outlines a comprehensive turn-key solution for creating and customizing a GMP production center, emphasizing a single point of contact for equipment to streamline project timelines and incorporate innovative technologies. It details various phases of construction and commissioning, qualification activities, and the establishment of quality systems, alongside training requirements for personnel. Additionally, it addresses process validation and the ongoing support provided to maintain facility quality and capacity after project completion.



![Process Validation
Objective
The objective of the Process Validation protocol is to demonstrate that [18F] FDG, using a
pre qualified equipment and materials, can be consistently manufactured to the required
product specification.
Scope
The PV protocol demonstrates that all aspects of [18F] FDG product quality are consistently
met when manufactured in accordance with the designated SOPs.
Furthermore during the PV these activities will be verified:
• Equipment/System qualification
• Product specifications
• Batch size and master formula
• Acceptance criteria
• Analytical method validation verification
• Stability test
• SOP’S verification
• Expiry testing
• Training Verification](https://image.slidesharecdn.com/1mpcomecer-140122050746-phpapp02/75/1MP-from-Comecer-17-2048.jpg)

