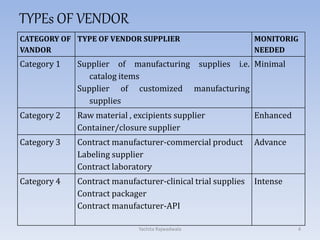
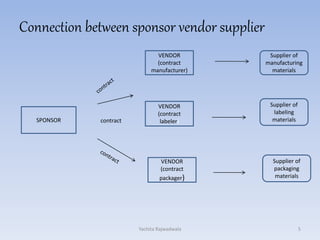
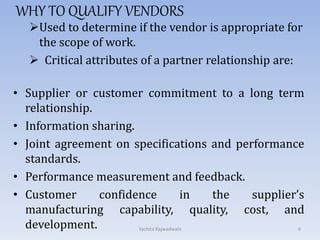
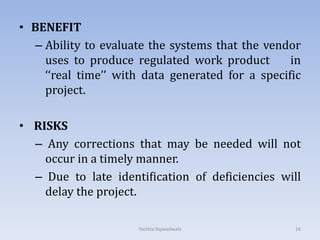
The document discusses vendor certification which involves qualifying vendors to ensure they will meet quality standards. It describes the certification process which includes selecting vendors, performing an on-site audit, reporting results, and follow up. Vendors are categorized based on the level of monitoring needed. The certification process benefits both companies by reducing costs and audits. Proper vendor qualification and ongoing monitoring is important for compliance with cGMP regulations.