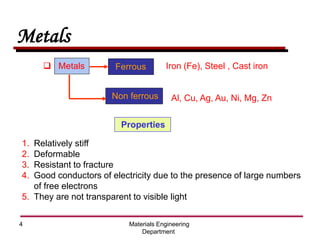
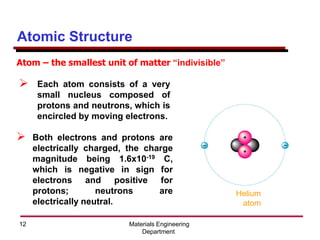
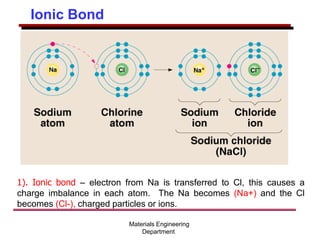
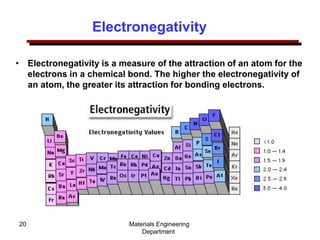
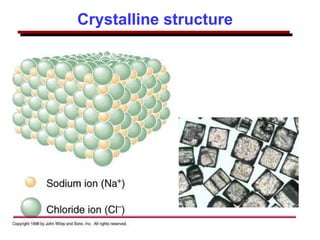
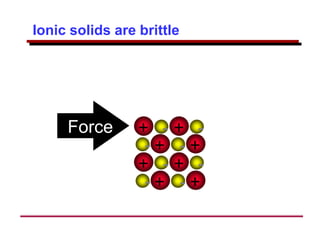
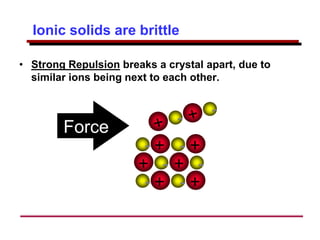
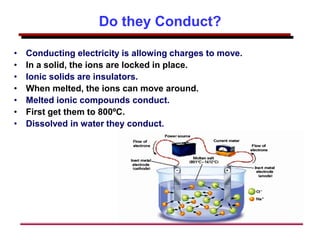
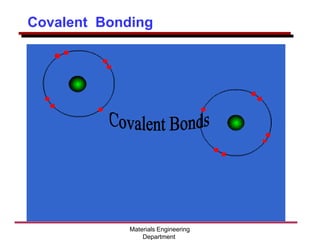
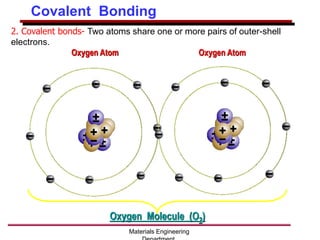
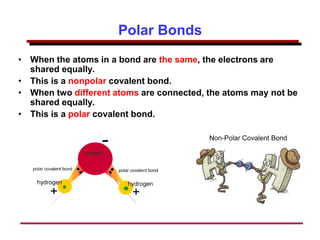
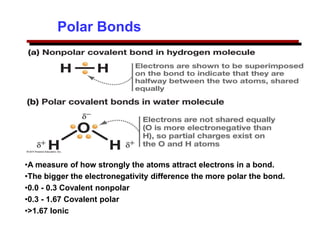
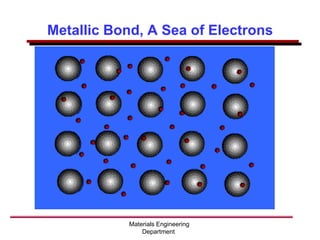
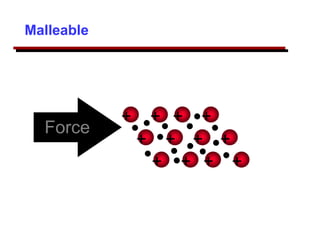
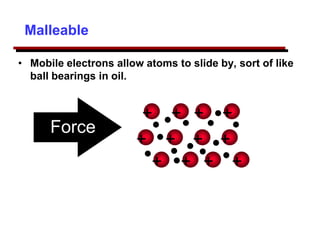
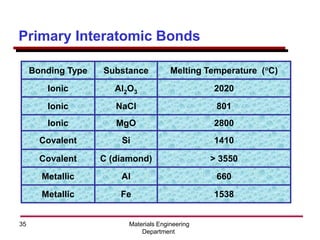
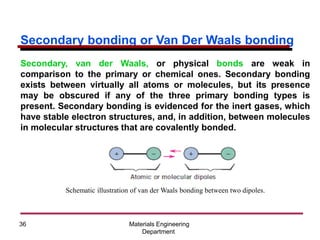
This document provides an introduction to engineering materials and their properties. It discusses the five major classes of materials - metals, ceramics, semiconductors, polymers, and composites. For each class, examples are given and their typical properties are described, such as metals being ductile and good conductors of electricity. The document also covers atomic structure, bonding types (ionic, covalent, metallic), and factors to consider for materials selection in engineering.