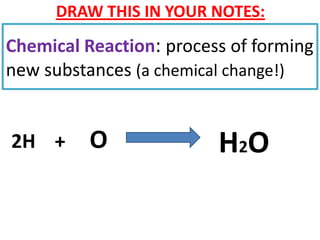
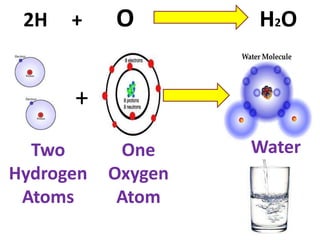
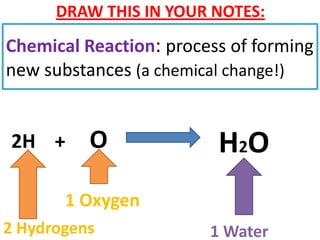
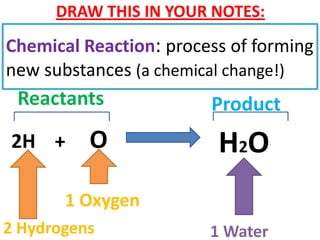
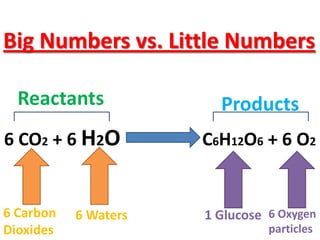
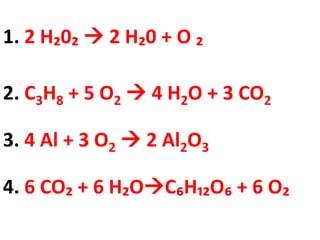
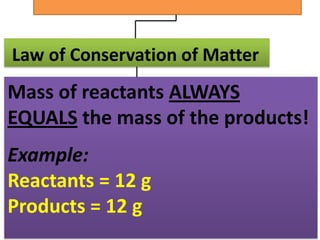
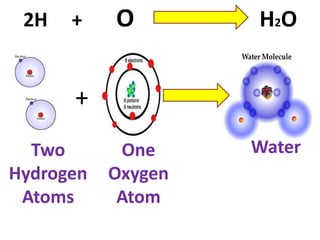
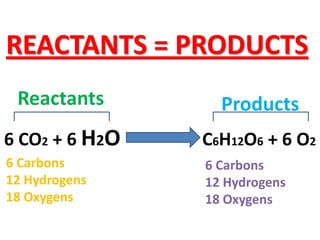
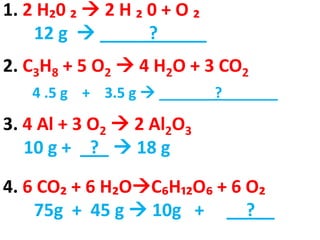
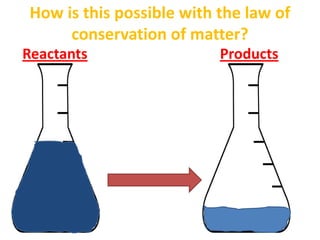
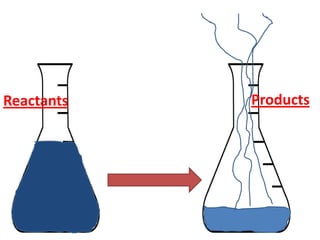
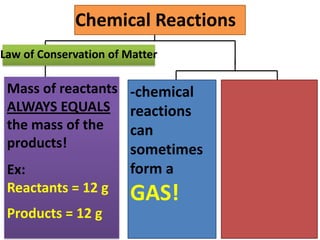
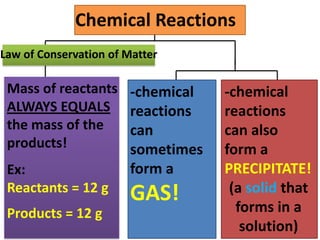
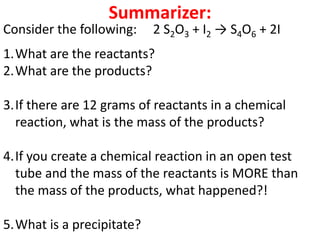
The document discusses chemical reactions, including identifying reactants and products, applying the law of conservation of matter stating the mass of reactants equals the mass of products, and recognizing that chemical reactions can form gases or precipitates. Reactants in the example reaction are S2O3 and I2, and products are S4O6 and 2I. If reactants are 12g, products are also 12g, and if reactants mass is more, a gas was likely formed. A precipitate is a solid that forms in a solution.