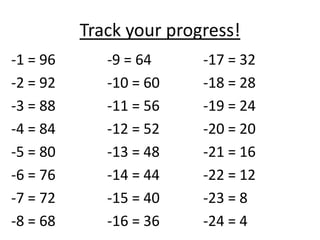
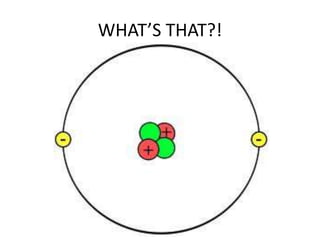
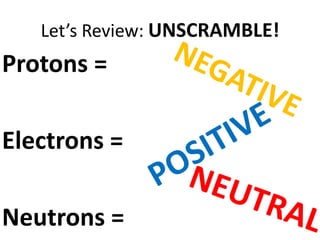
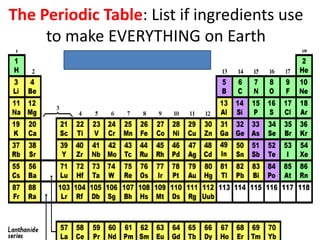
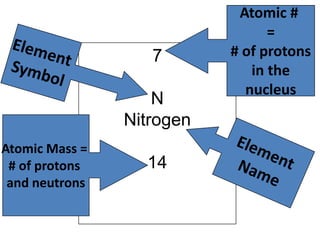
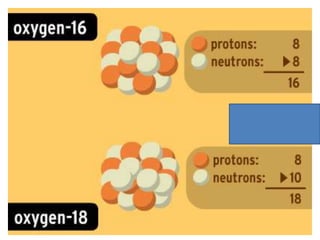
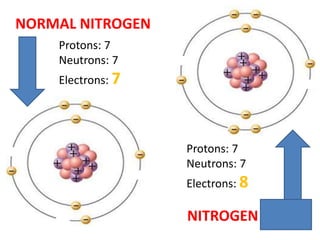
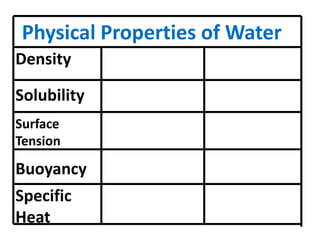
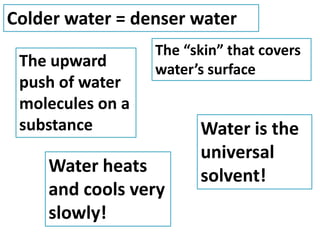
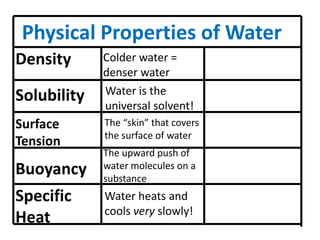
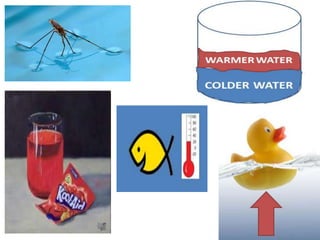
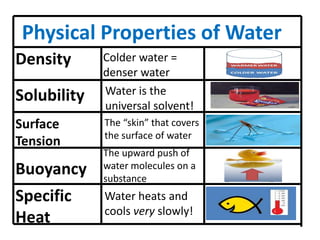
Water has several unique physical properties that are important for life. Its high specific heat means water can absorb large amounts of heat without a large temperature change, allowing bodies of water to moderate temperatures. Water also has a density maximum at 4°C, causing it to expand upon freezing which is unusual for liquids. Additionally, water has a high surface tension and solvent properties, enabling organisms to float, insects to walk on water, and allowing for the dissolution of many substances important for biological functions. These combined physical properties make water well-suited for life.