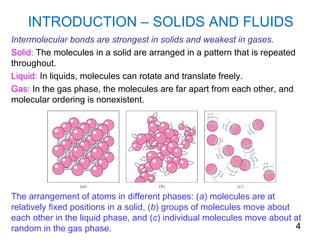
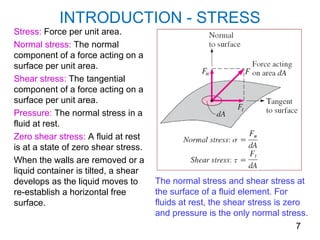
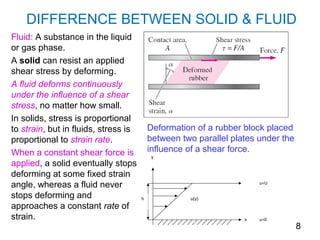
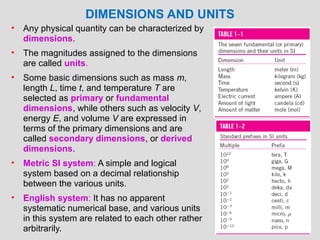
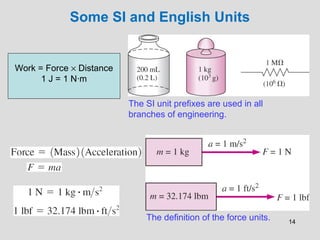
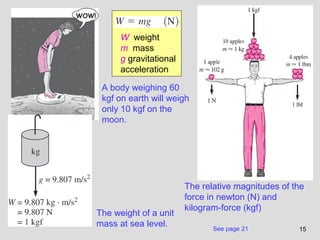
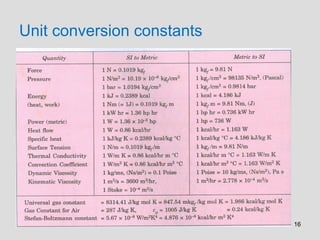
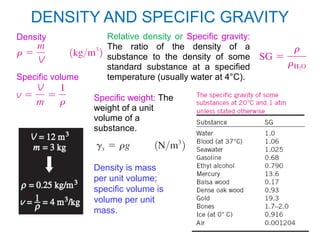
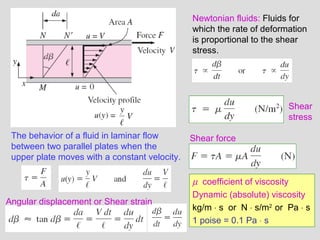
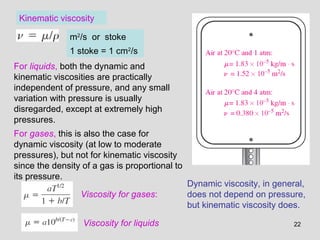
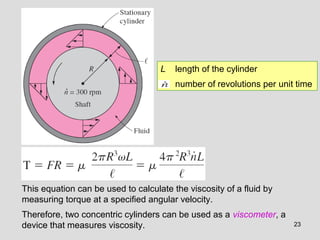
The document provides an introduction to fluid mechanics, including key concepts, applications, dimensions and units, and properties of fluids. It defines fluids, fluid statics and dynamics, stress, density, viscosity, and introduces various units of measurement. Viscosity is further explained, noting it represents resistance to flow and is measured using devices like concentric cylinder viscometers. Applications include areas like artificial hearts. Dimensional analysis helps characterize physical quantities.