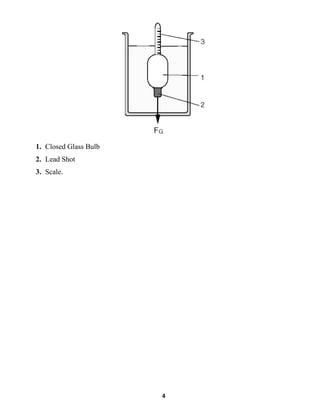
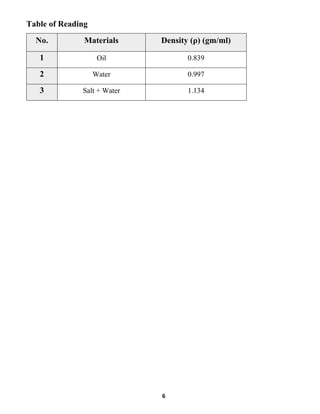
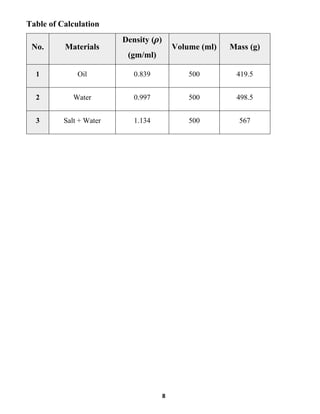
This document summarizes a fluid mechanics laboratory experiment to determine liquid density using an aerometer or hydrometer. The experiment involved measuring the density of oil, water, and a saltwater mixture by placing aerometers of varying scales into each liquid and reading the line where they floated. Densities of 0.839 g/ml for oil, 0.997 g/ml for water, and 1.134 g/ml for saltwater were obtained. The mass of each liquid was then calculated using the measured density and a volume of 500ml. The location of the lead shot in the bottom of the aerometer allows it to right itself vertically when measuring density by balancing the lifting and gravitational forces.







![10
References
1. Tatum M., 2020. What is an Aerometer?. [online] Wisegeek. Available at:
https://www.wisegeek.com/what-is-an-aerometer.htm [Accessed 9 NOV. 2020].
2. Avogadro's Lab Supply, Inc. (2019). How To Use A Hydrometer. [online] Available at:
https://www.avogadro-lab supply.com/content/How_To_Use_A_Hydrometer/2
[Accessed 9 NOV. 2020].](https://image.slidesharecdn.com/fluidmechanicslabexperiment02aerometerhydrometer-210319141607/85/Fluid-mechanics-lab-experiment-02_aerometer_hydrometer-12-320.jpg)