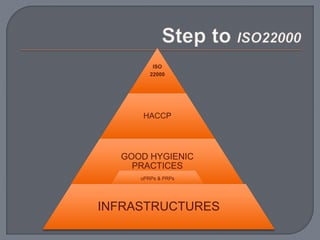
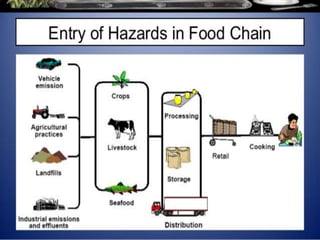
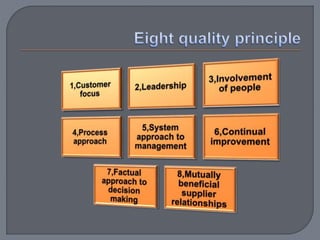
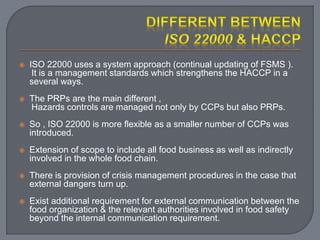
The International Organization for Standardization (ISO), founded in 1947 and based in Geneva, develops standards such as ISO 22000 for food safety management systems, applicable to all sectors of the food industry. It bridges public and private sectors, promoting safe food practices and compliance through structured systems and guidelines, including prerequisites and operational measures. ISO 22000 allows businesses to demonstrate their commitment to food safety, enhance consumer confidence, and improve overall operational efficiency.