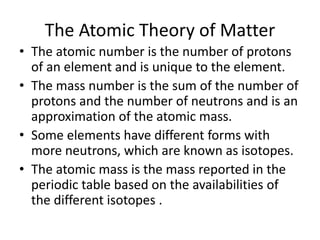
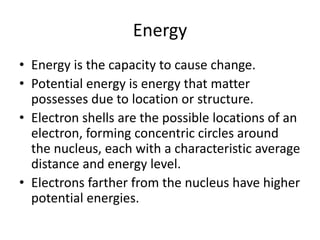
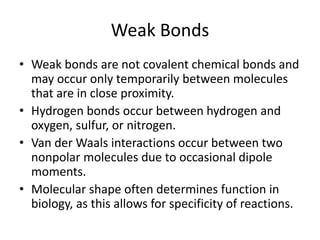
The Scientific Method involves making observations, forming hypotheses, experimentation, analyzing results, and making conclusions. It is the process scientists use to make discoveries about natural phenomena. The key steps are:
1) Making observations to collect qualitative and quantitative data
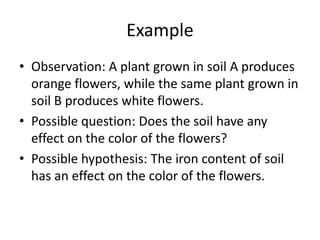
2) Forming questions from observations and proposing hypotheses as tentative answers
3) Experimentation to test hypotheses
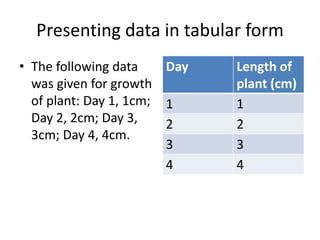
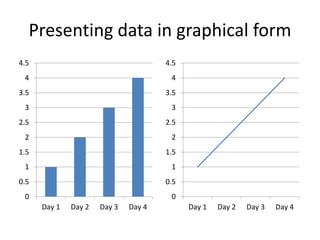
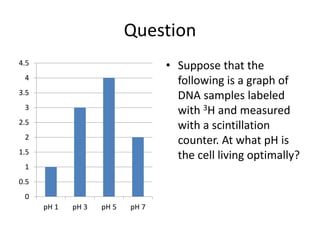
4) Analyzing results, which can be presented in tables or graphs
5) Drawing conclusions based on the experimental data