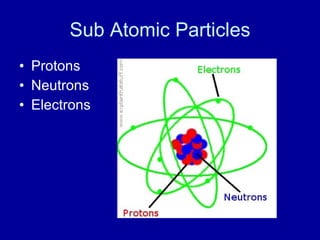
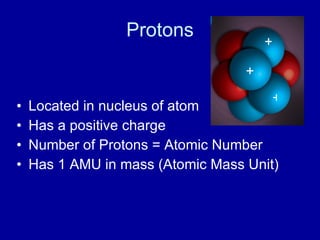
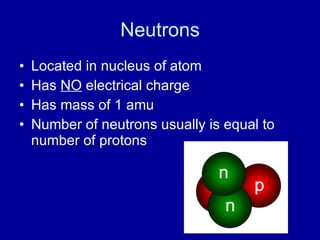
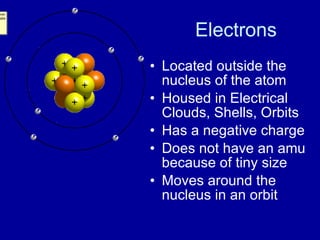
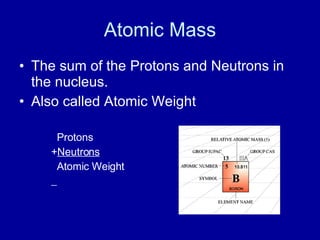
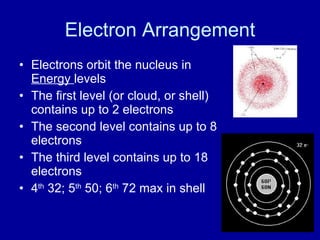
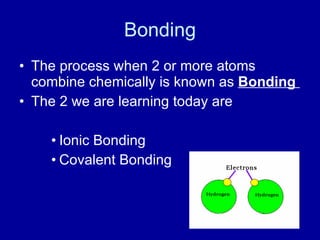
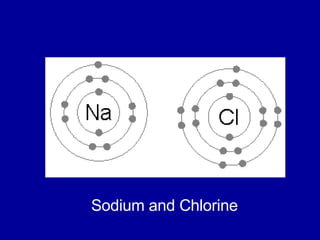
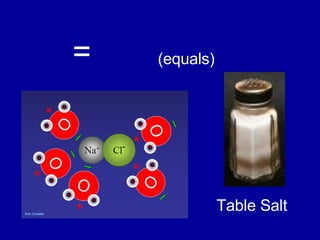
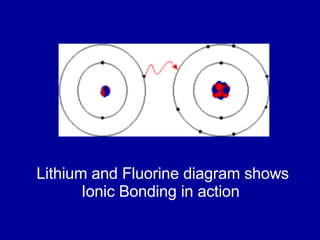
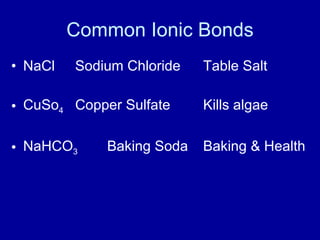
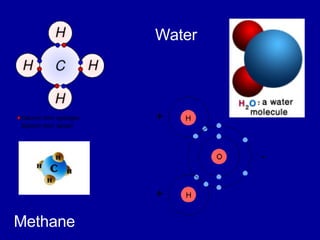
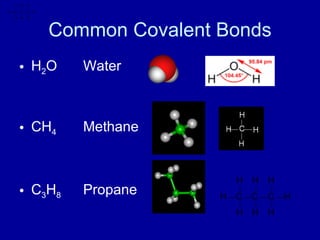
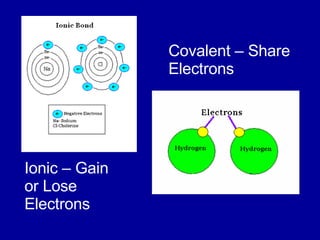
This document provides an overview of atomic structure and bonding. It discusses subatomic particles like protons, neutrons, and electrons and their properties. It explains how atomic number and mass are determined. Electron arrangement in energy levels is covered, along with ionic and covalent bonding processes. Ionic bonding occurs through the transfer of electrons between atoms, while covalent bonding involves the sharing of electrons between atoms. Common ionic and covalent compounds are listed as examples.