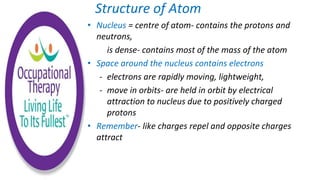
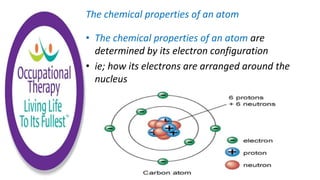
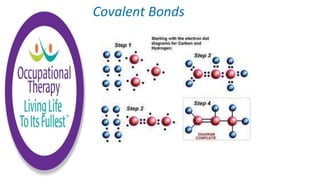
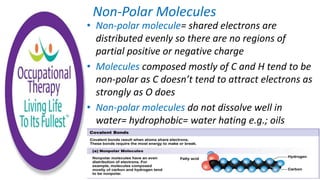
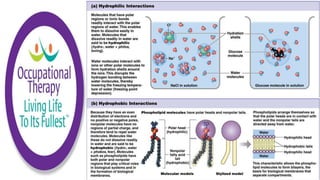
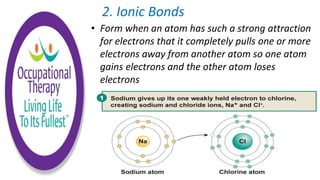
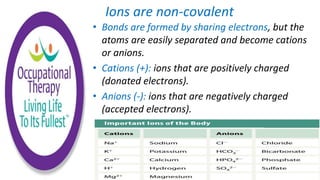
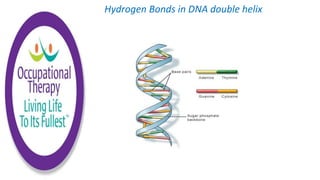
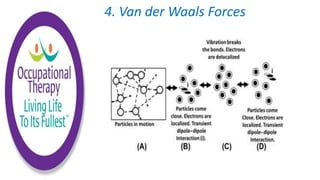
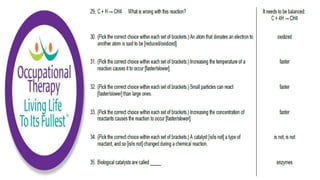
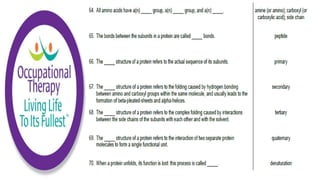
Human physiology involves the study of molecules, cells, tissues, organs and organ systems that make up the human body. At the most basic level, atoms combine through chemical bonds like ionic and covalent bonds to form molecules, which then organize into cells. Cells further organize into tissues and organs to carry out specific functions and form organ systems that allow the human body to function as a whole.