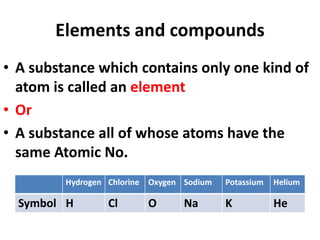
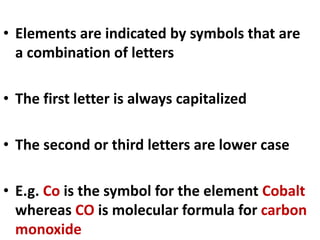
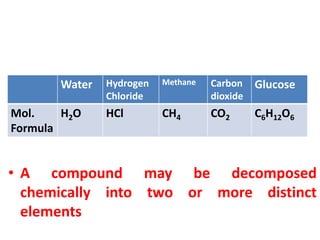
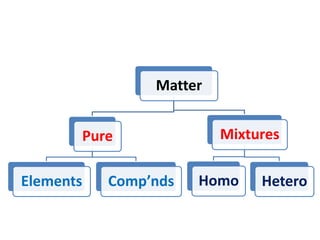
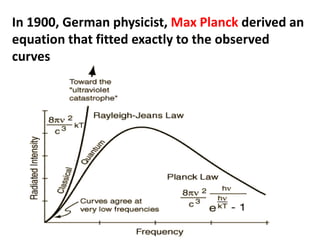
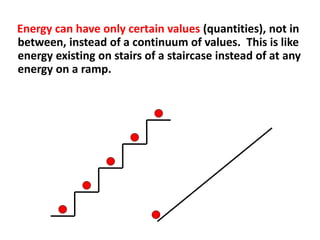
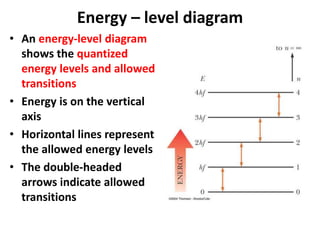
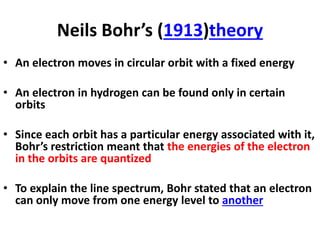
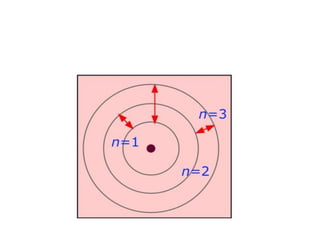
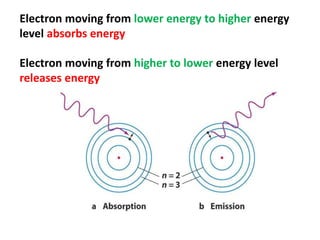
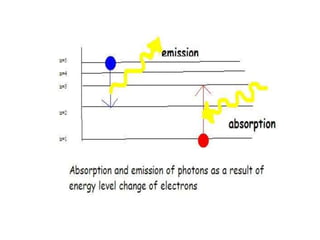
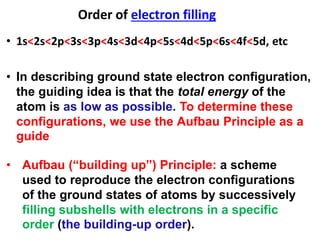
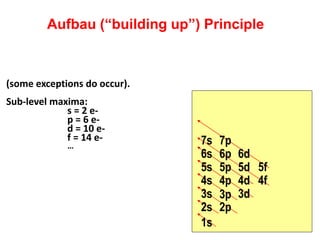
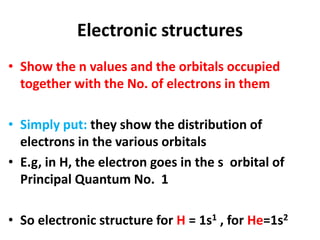
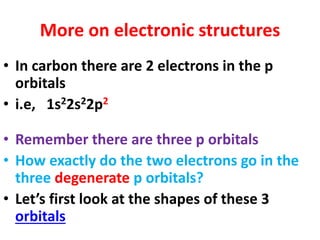
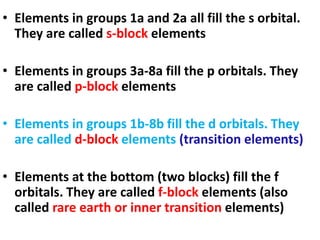
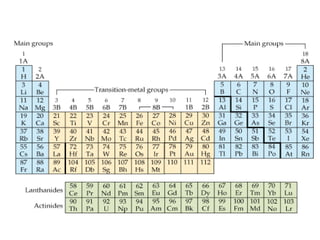
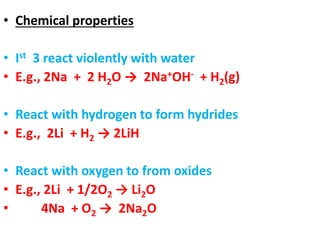
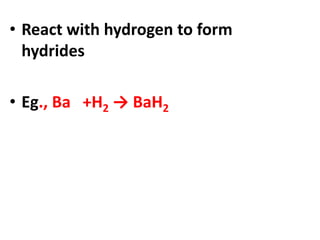
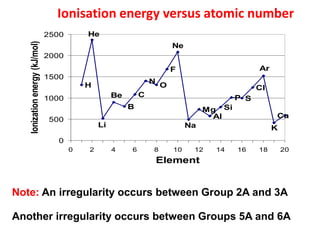
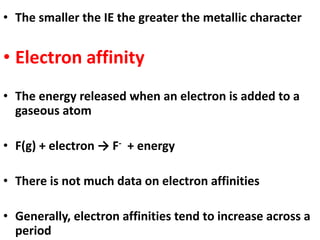
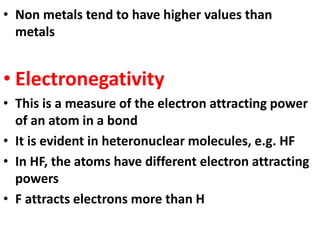
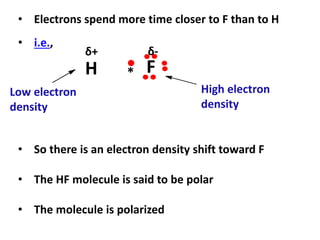
This document provides information about chemistry concepts including the atomic theory, structure of the atom, atomic mass units, atomic weights, elements, compounds, and pure substances vs mixtures. Key points include:
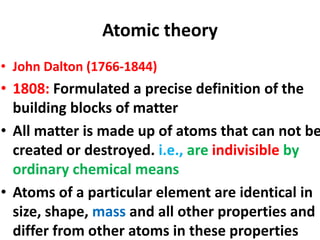
- John Dalton formulated the atomic theory including that atoms are indivisible and differ between elements.
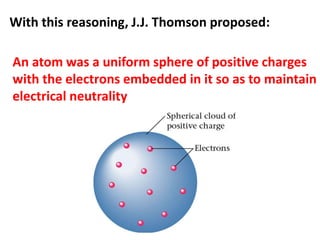
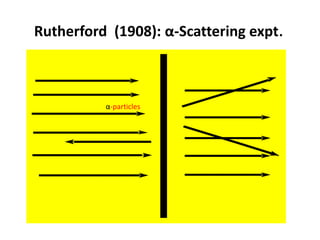
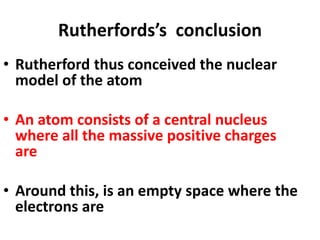
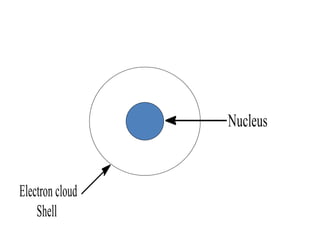
- Rutherford discovered the nuclear model of the atom with a small, dense nucleus and electrons in empty space around it.
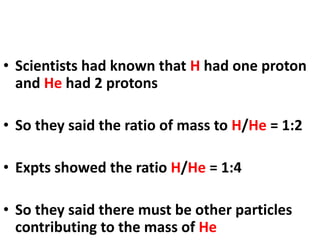
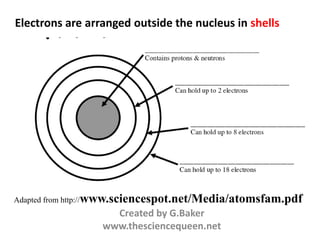
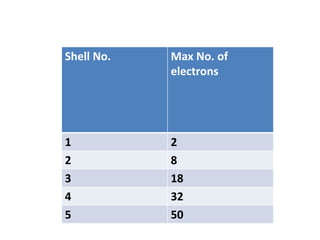
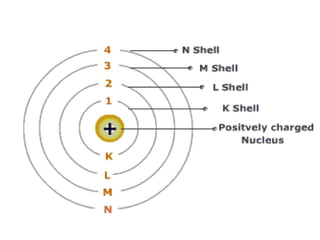
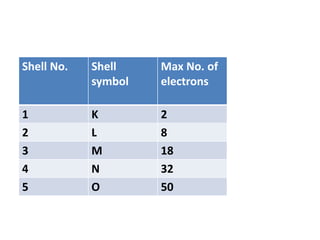
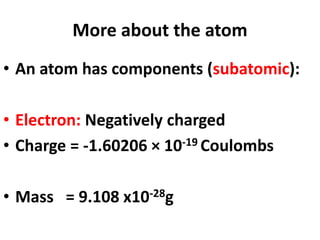
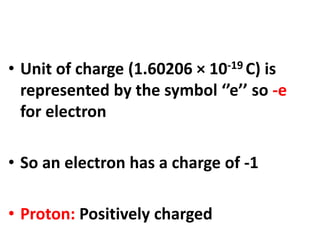
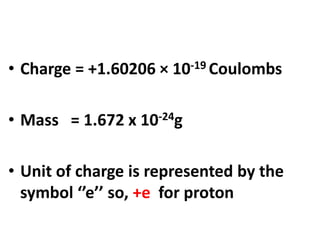
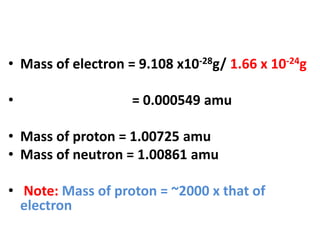
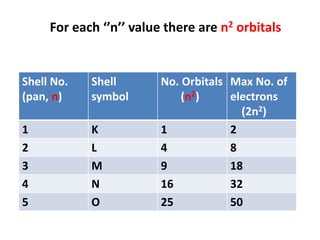
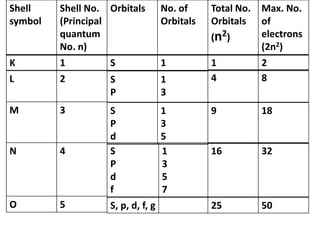
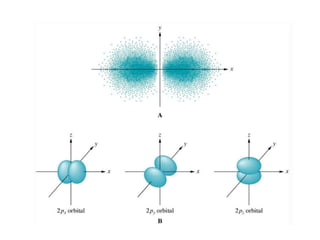
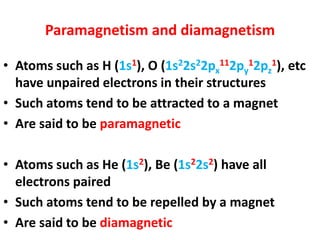
- Atoms contain protons, neutrons, and electrons. Electrons are arranged in shells around the nucleus.
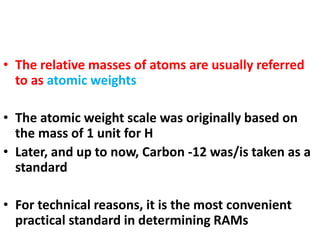
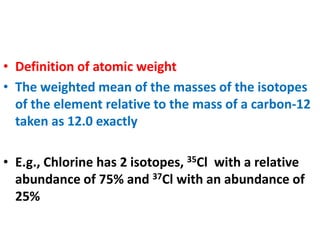
- Atomic mass units are used to express atomic masses rather than grams due to atoms' small size. Atomic weights are relative masses of atoms compared to carbon-12.