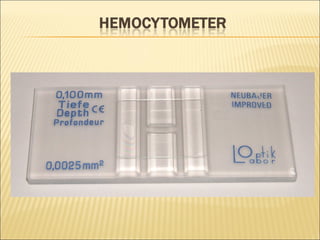
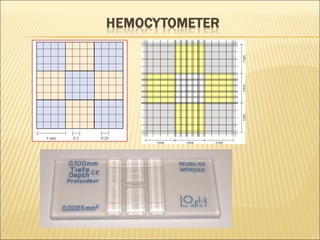
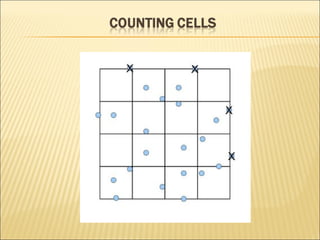
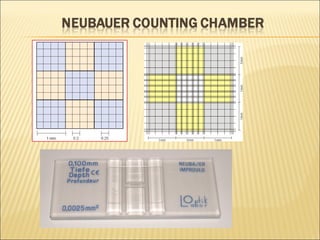
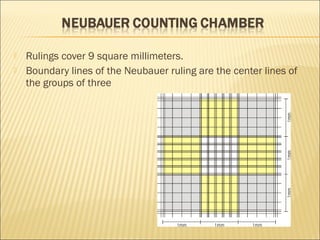
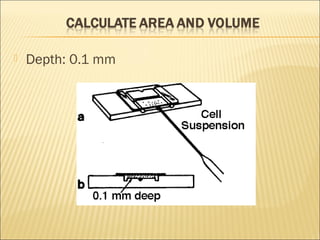
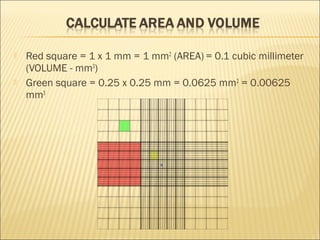
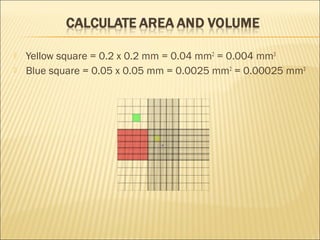
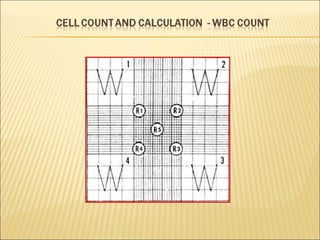
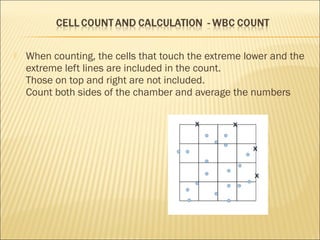
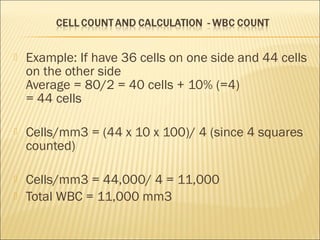
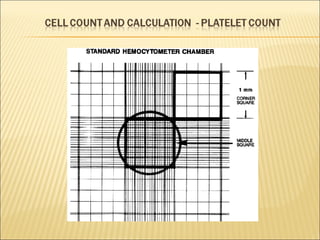
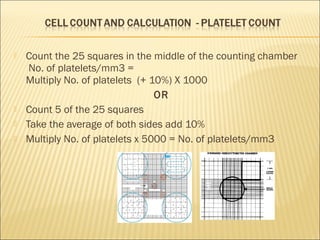
The document describes how to use a hemocytometer to count cells in a liquid sample. A hemocytometer has a chamber of known volume and depth that allows counting cells within a defined area to calculate concentration. Proper sample preparation is important, such as ensuring an appropriate cell concentration that is not too high or low. Cells are counted within the grid lines and their number is used to calculate the concentration of cells in the original sample volume.