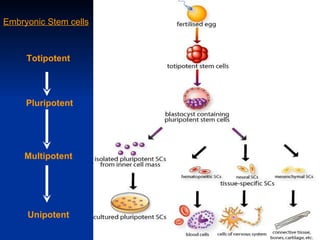
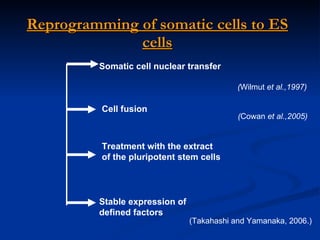
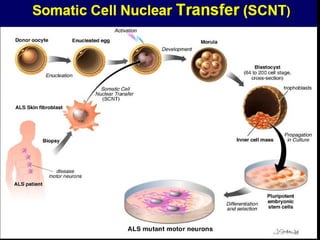
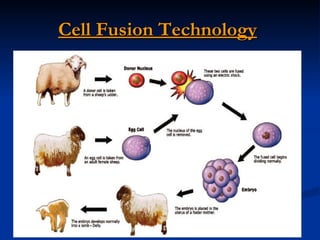
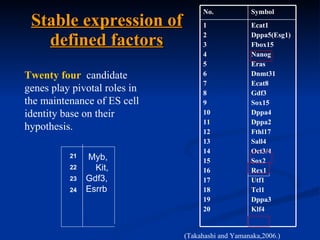
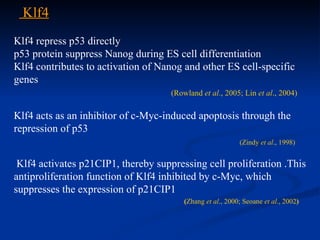
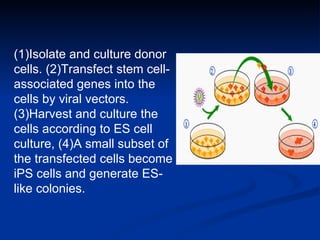
The document discusses induced pluripotent stem cells (iPSCs), which are derived from adult somatic cells that are reprogrammed by introducing genes associated with pluripotency. iPSCs were first generated in 2006 and resemble embryonic stem cells. They can be produced from a person's own cells and have potential applications in disease modeling, drug development, and regenerative medicine without ethical issues associated with embryonic stem cells.