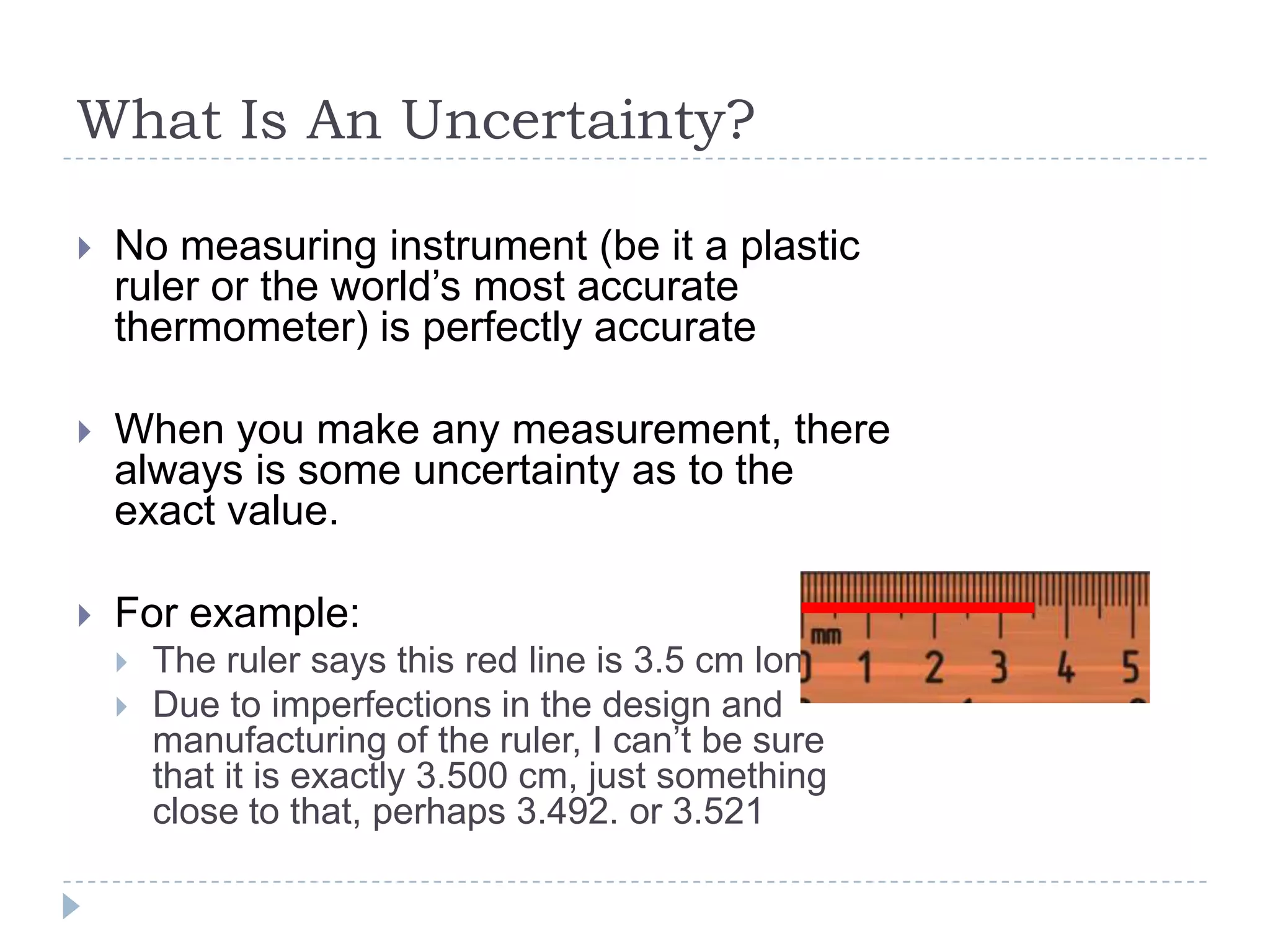
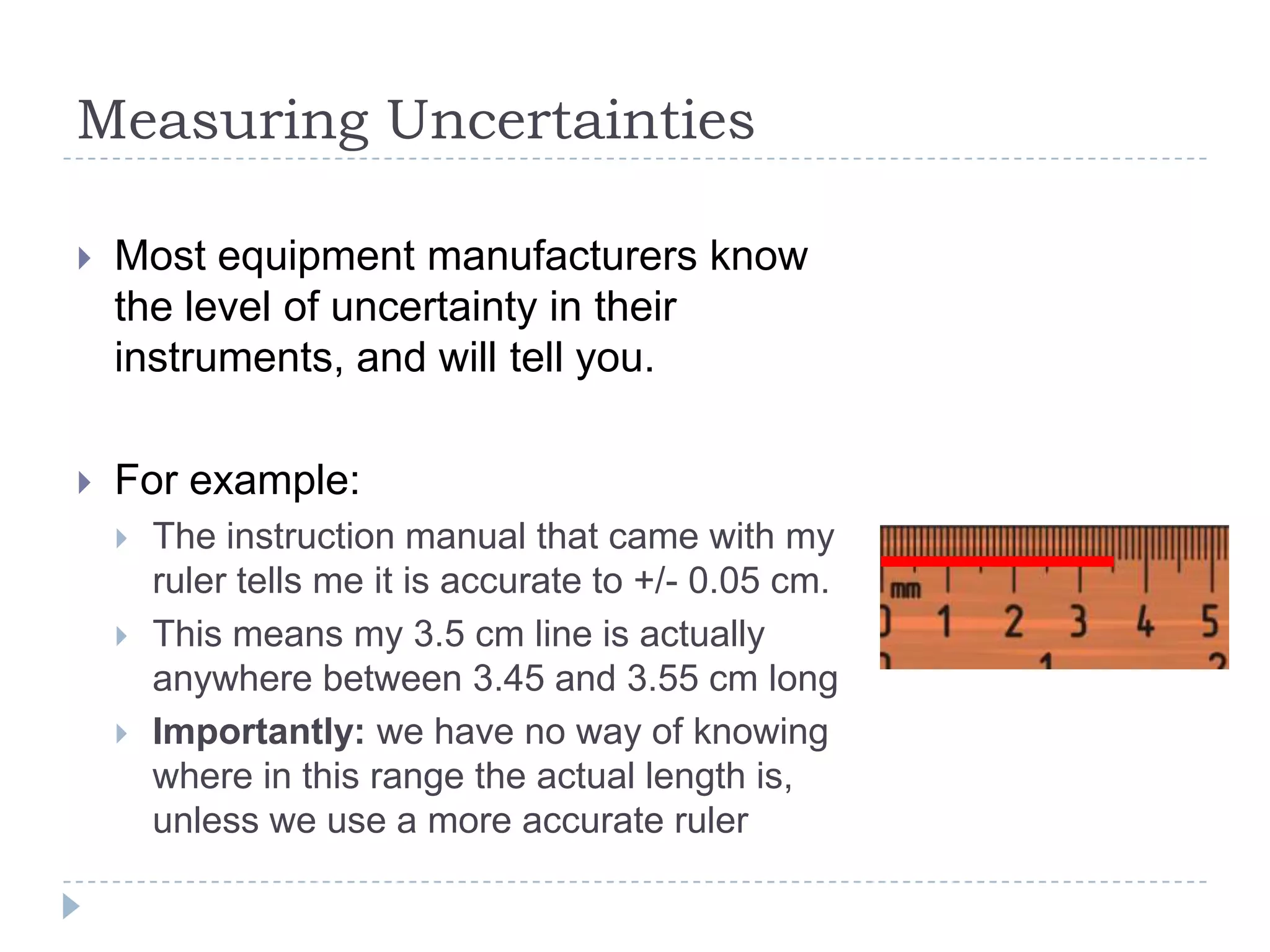
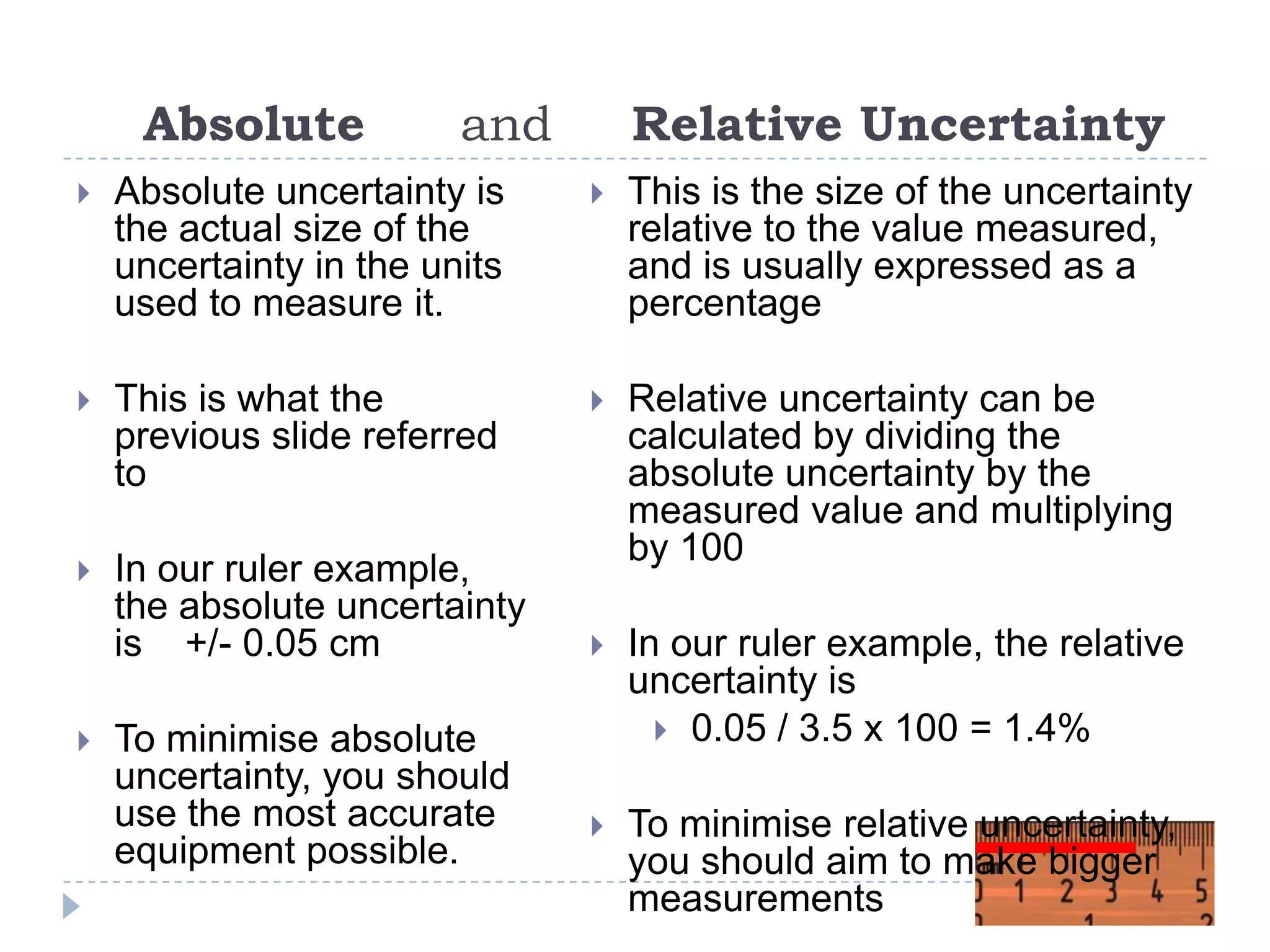
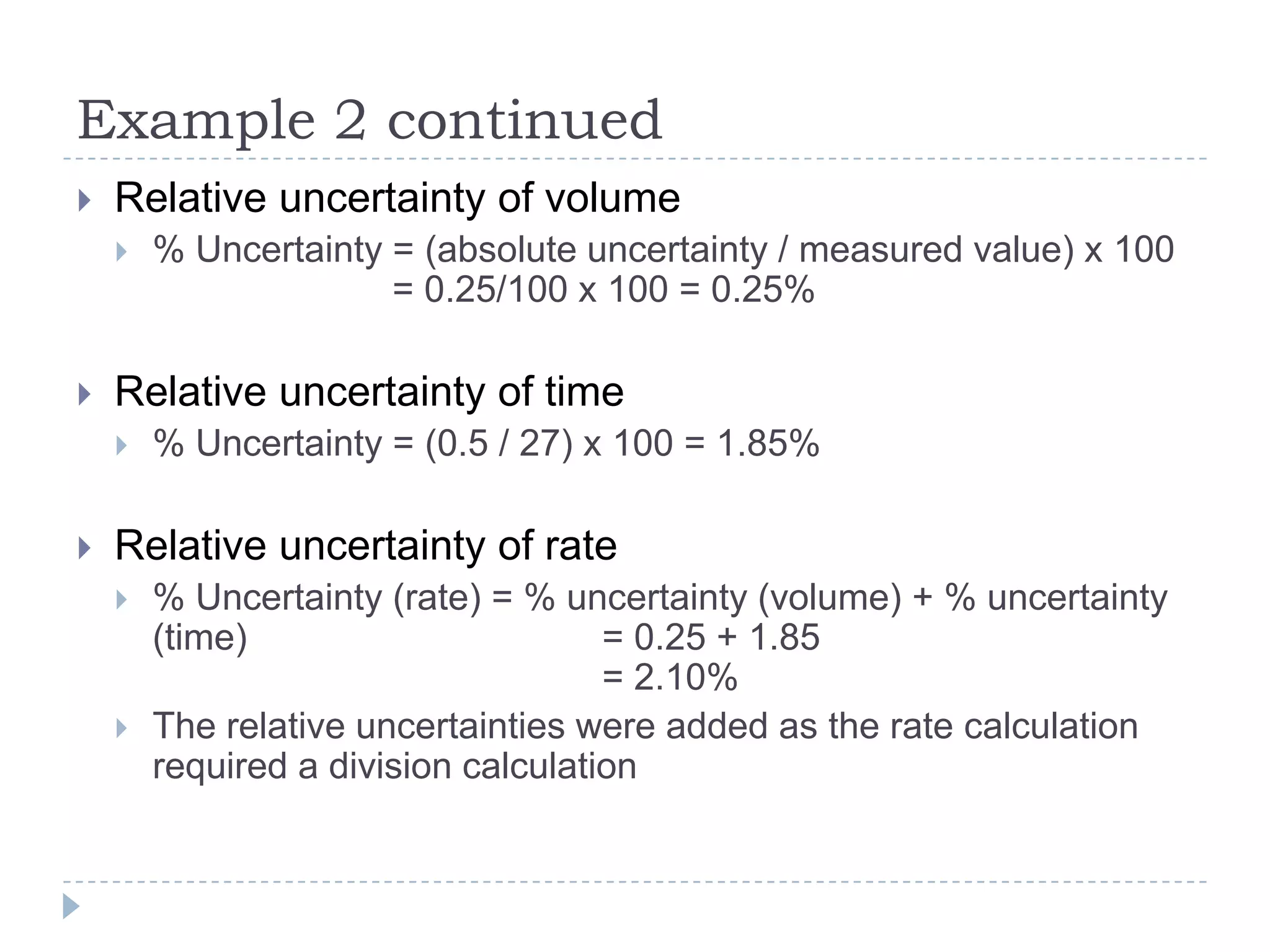
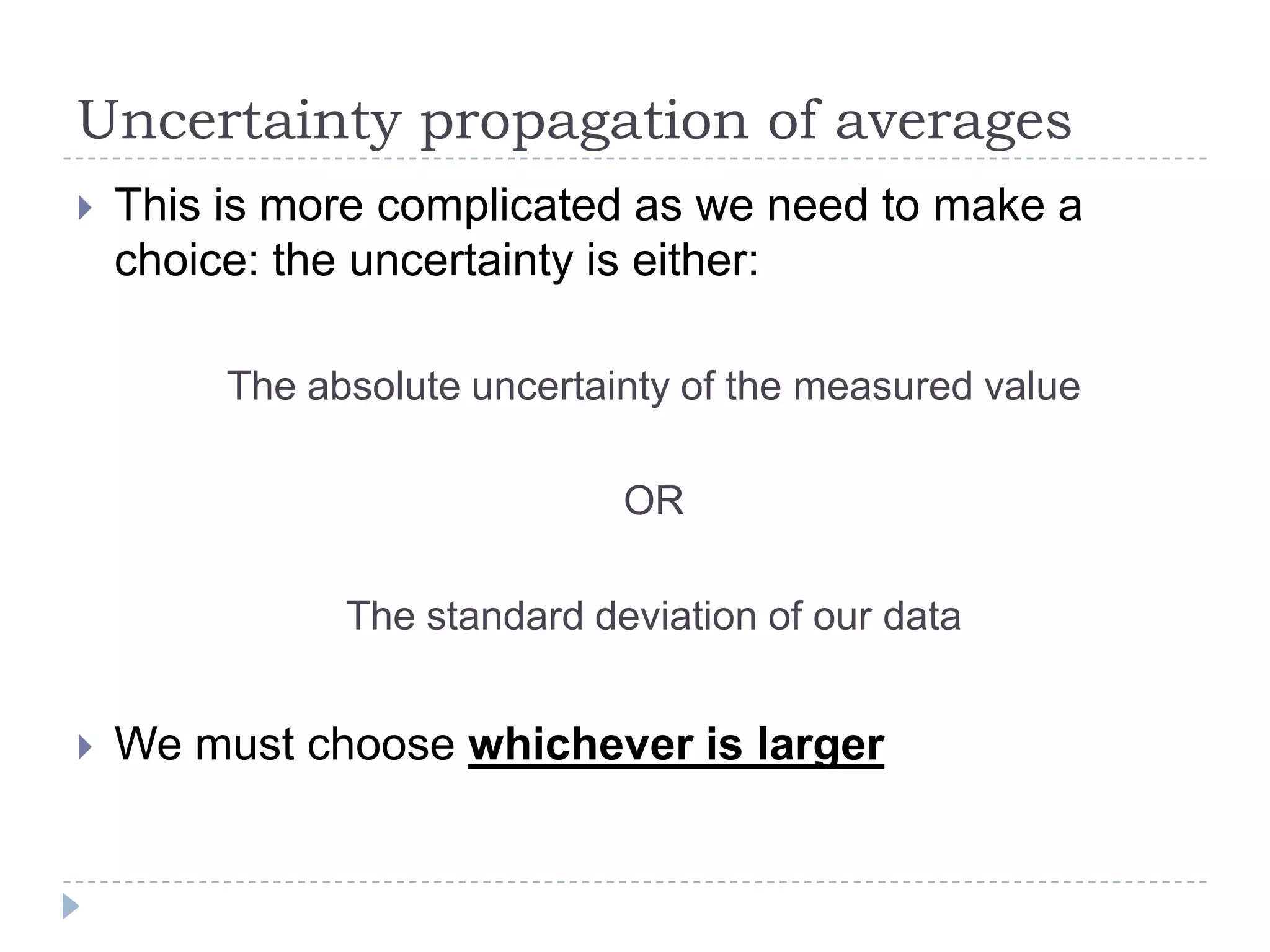
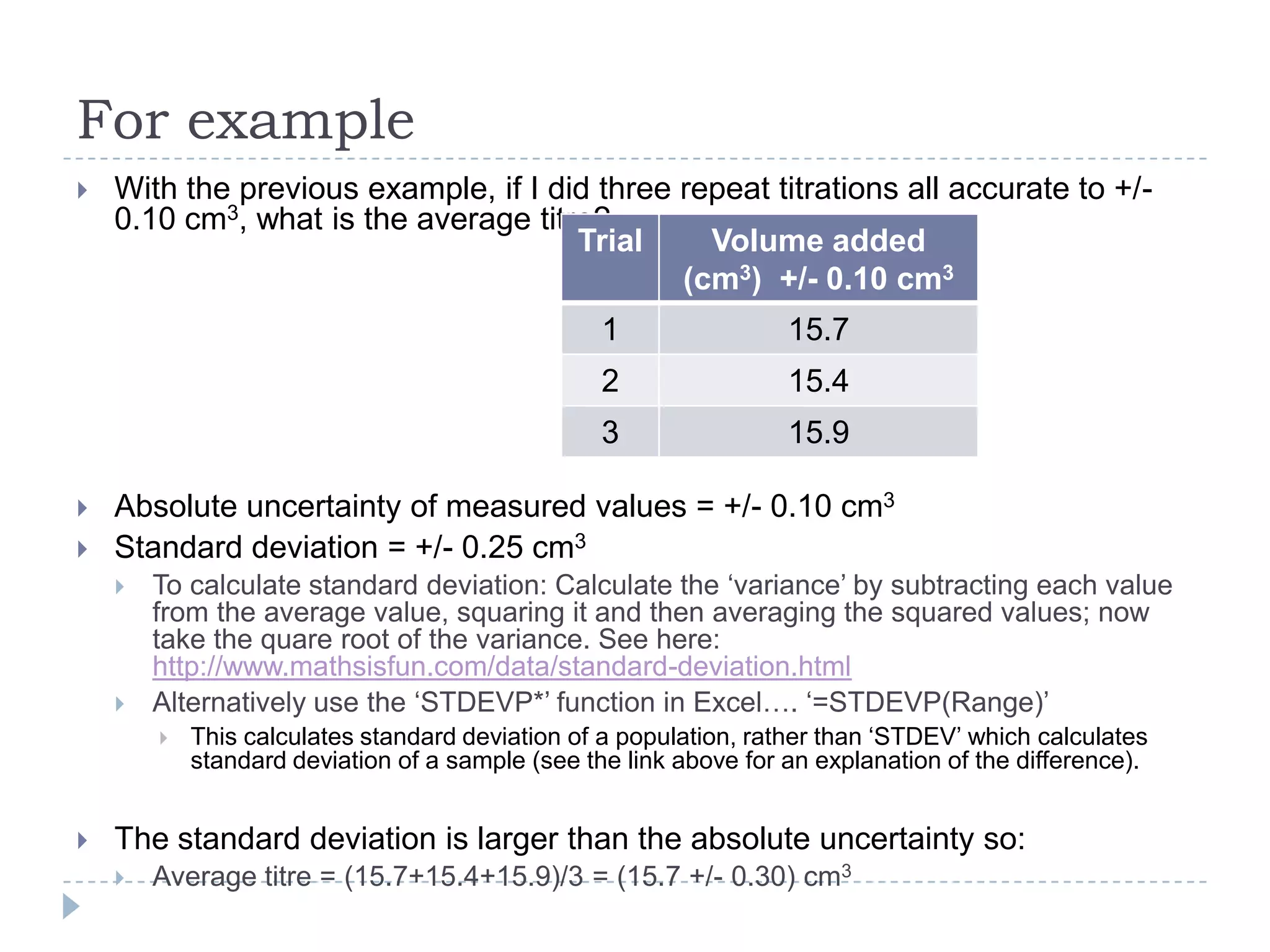
The document provides a concise guide on calculating uncertainties in measurements, explaining the concepts of absolute and relative uncertainty. It includes practical examples, such as measuring volume and reaction rates, and describes how to propagate uncertainty through calculations. The guide emphasizes the importance of knowing the uncertainty of measuring instruments to enhance accuracy in scientific experiments.