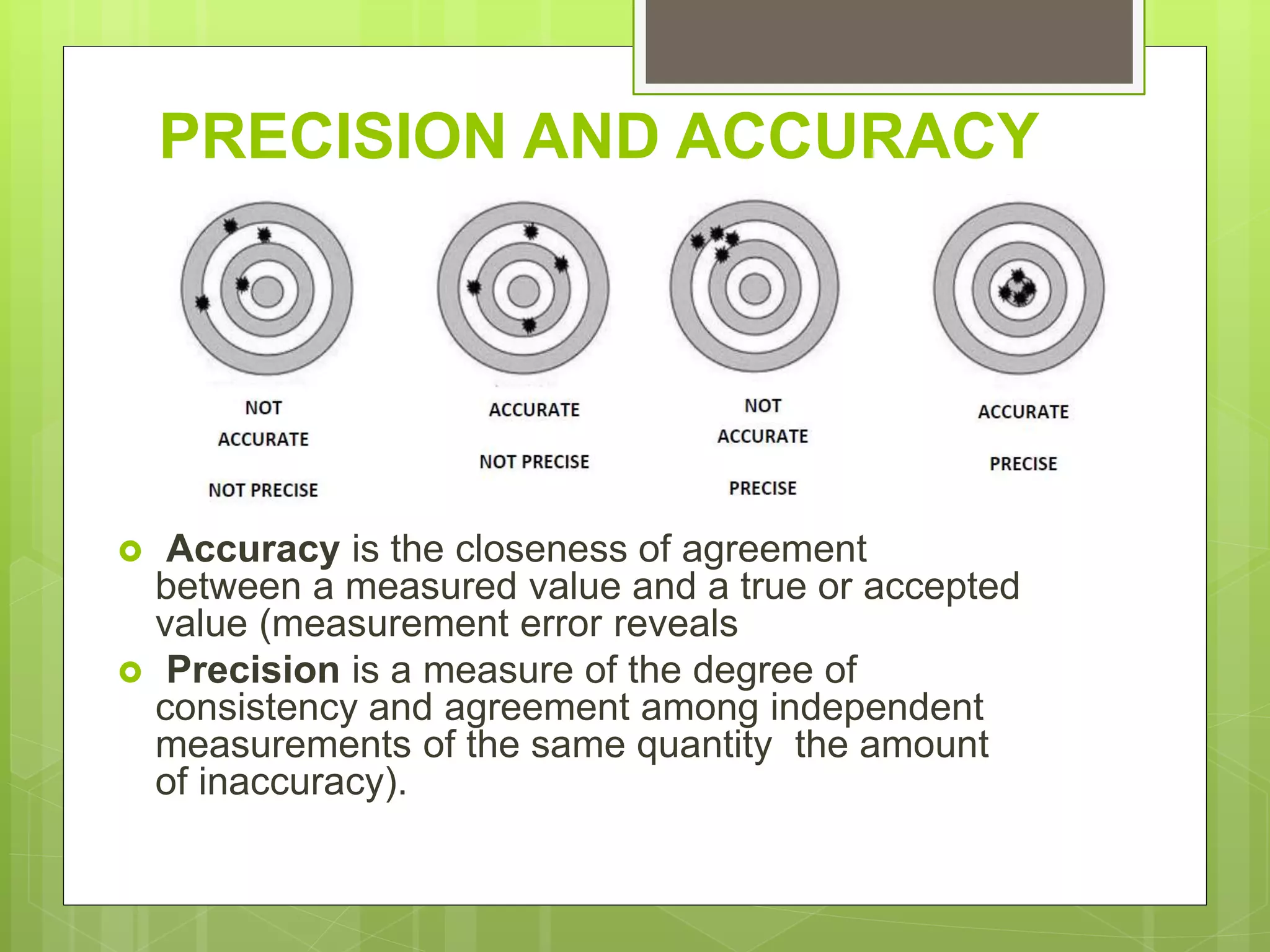
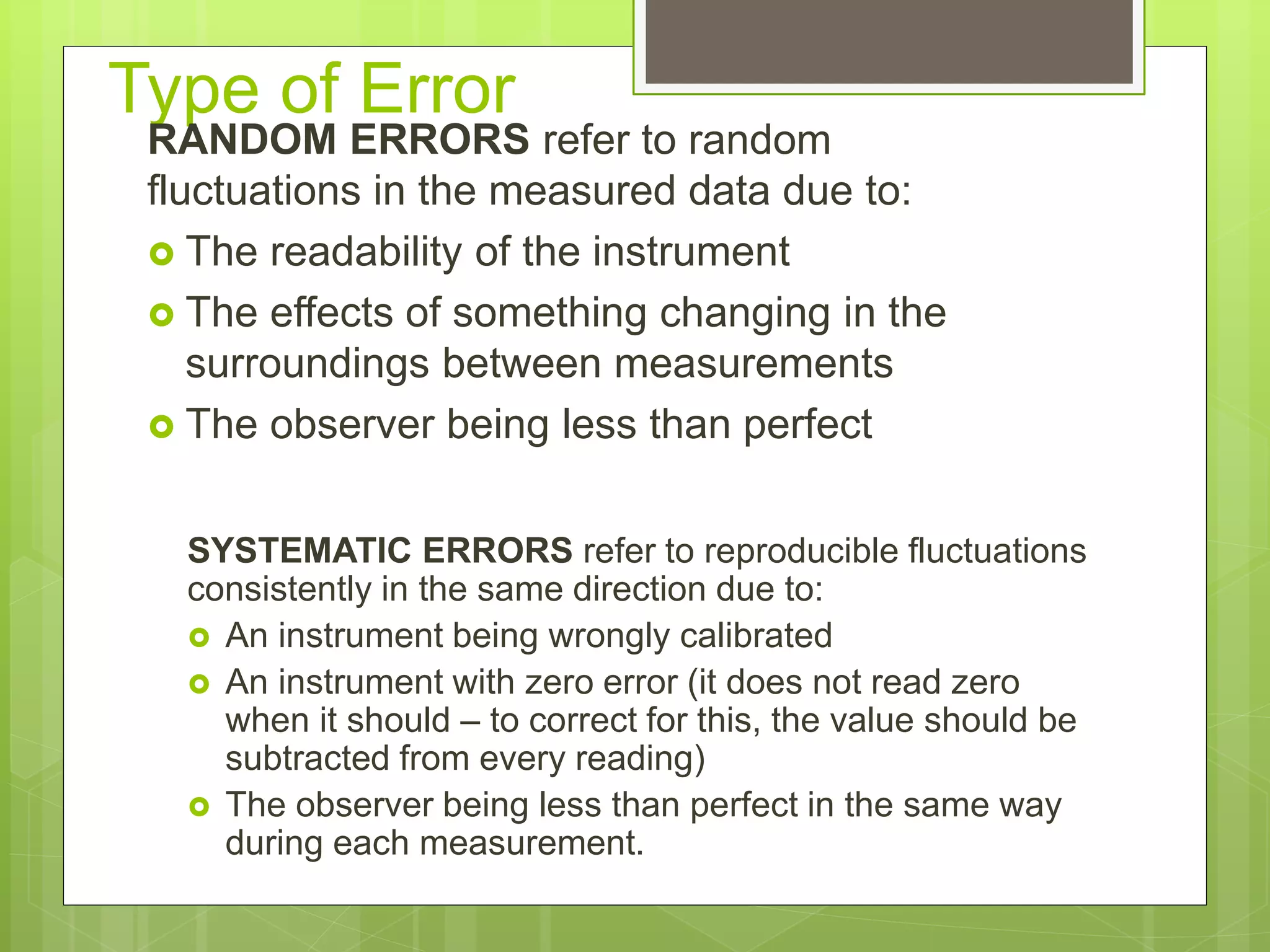
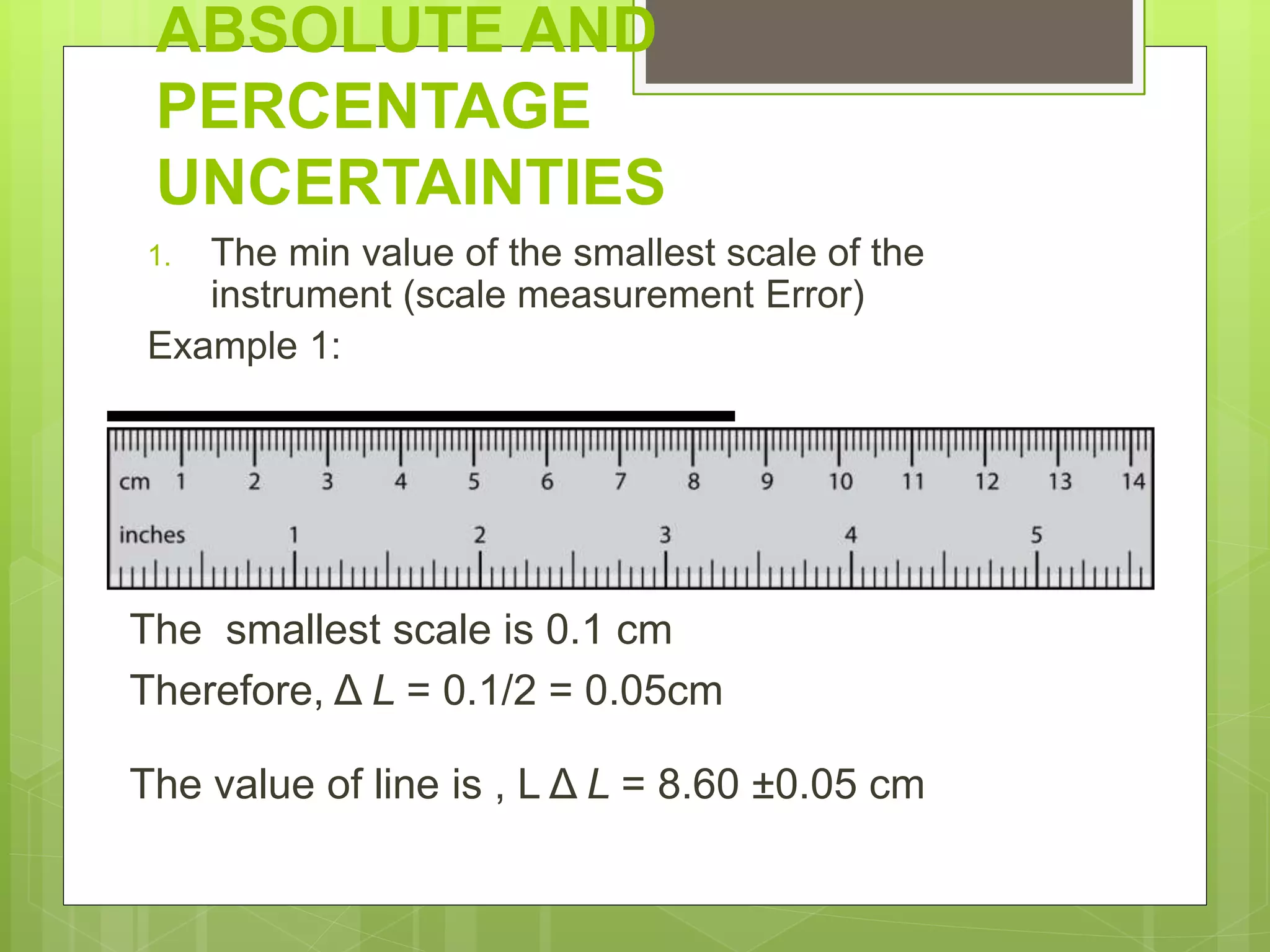
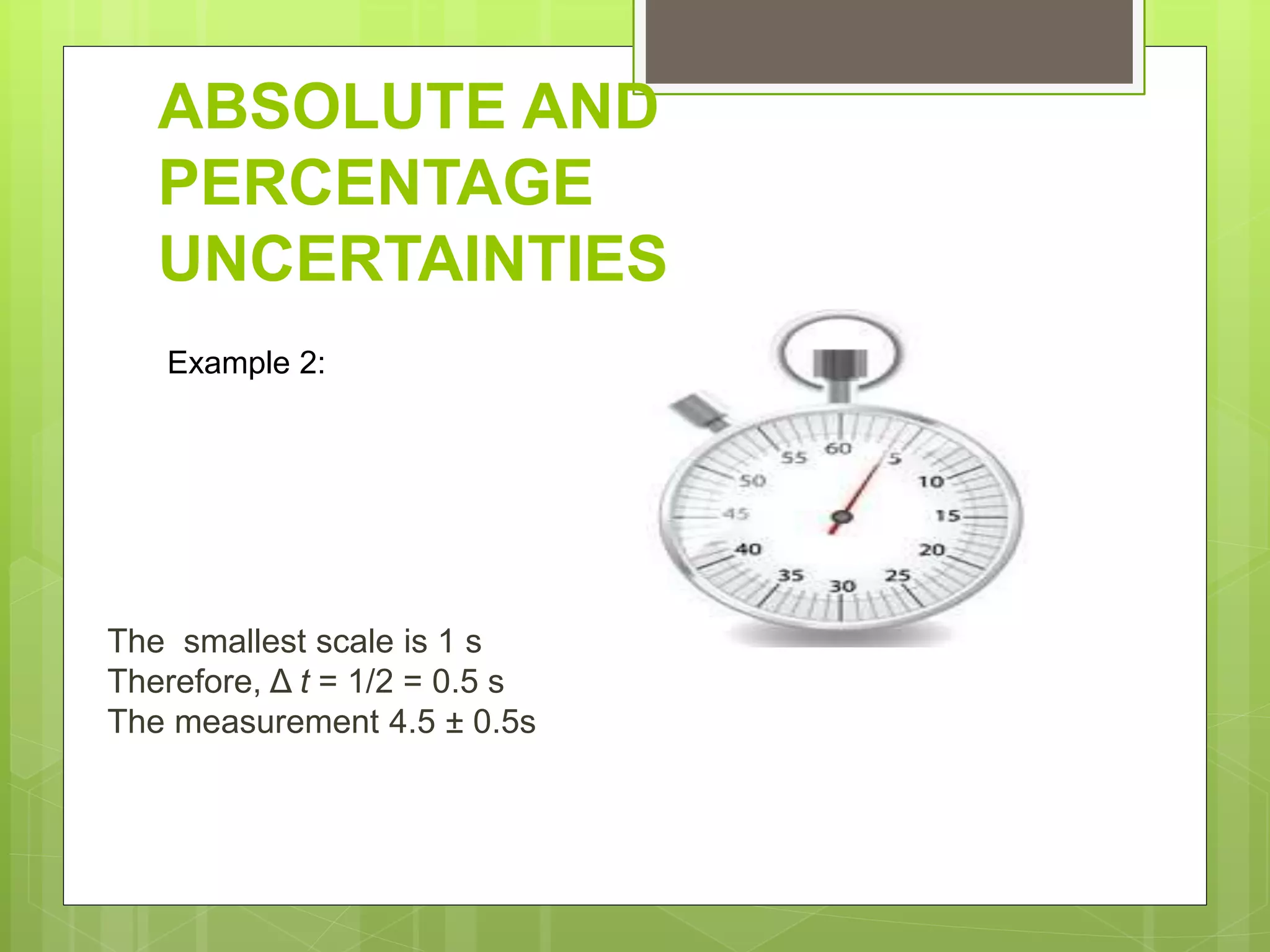
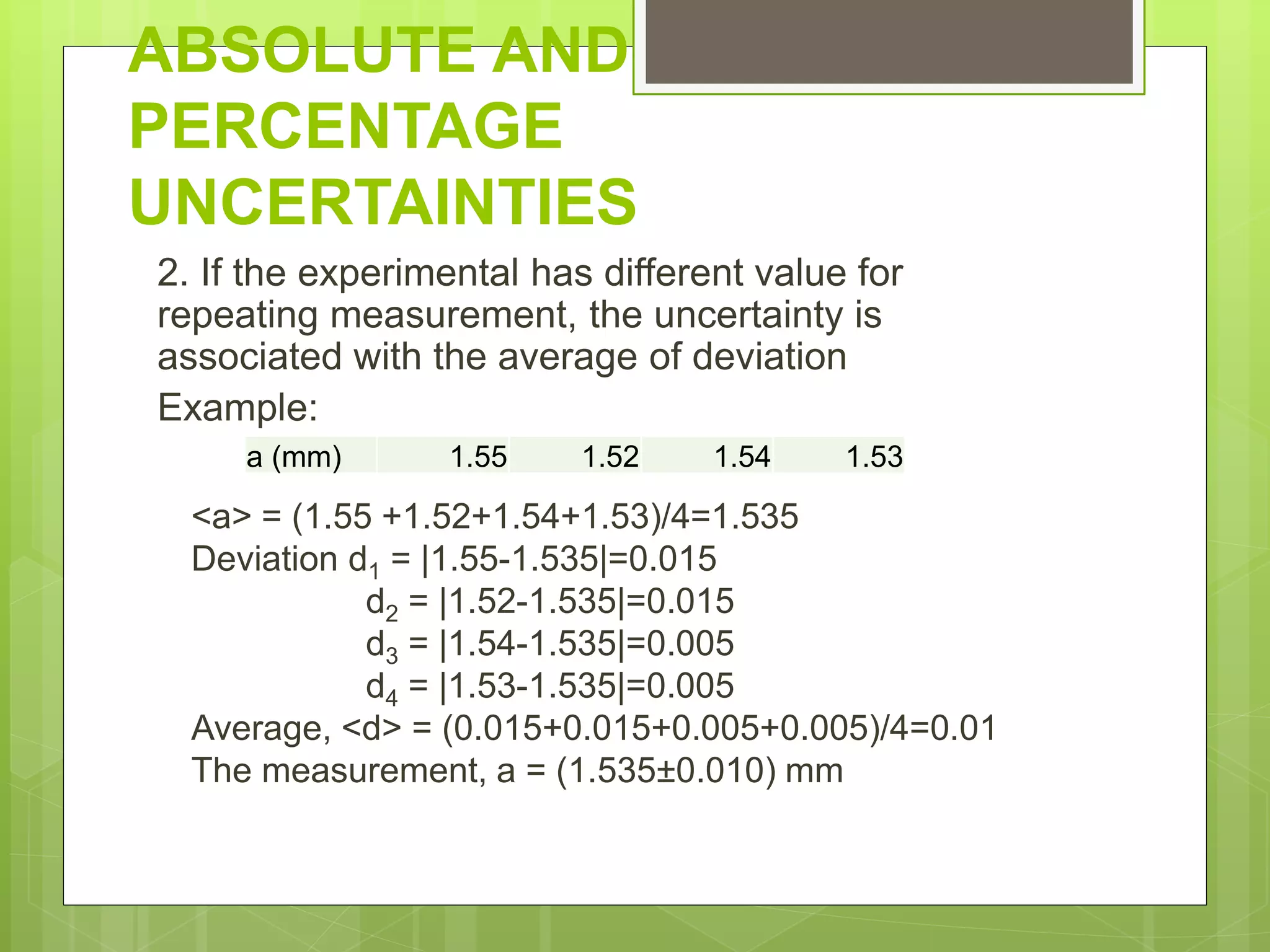
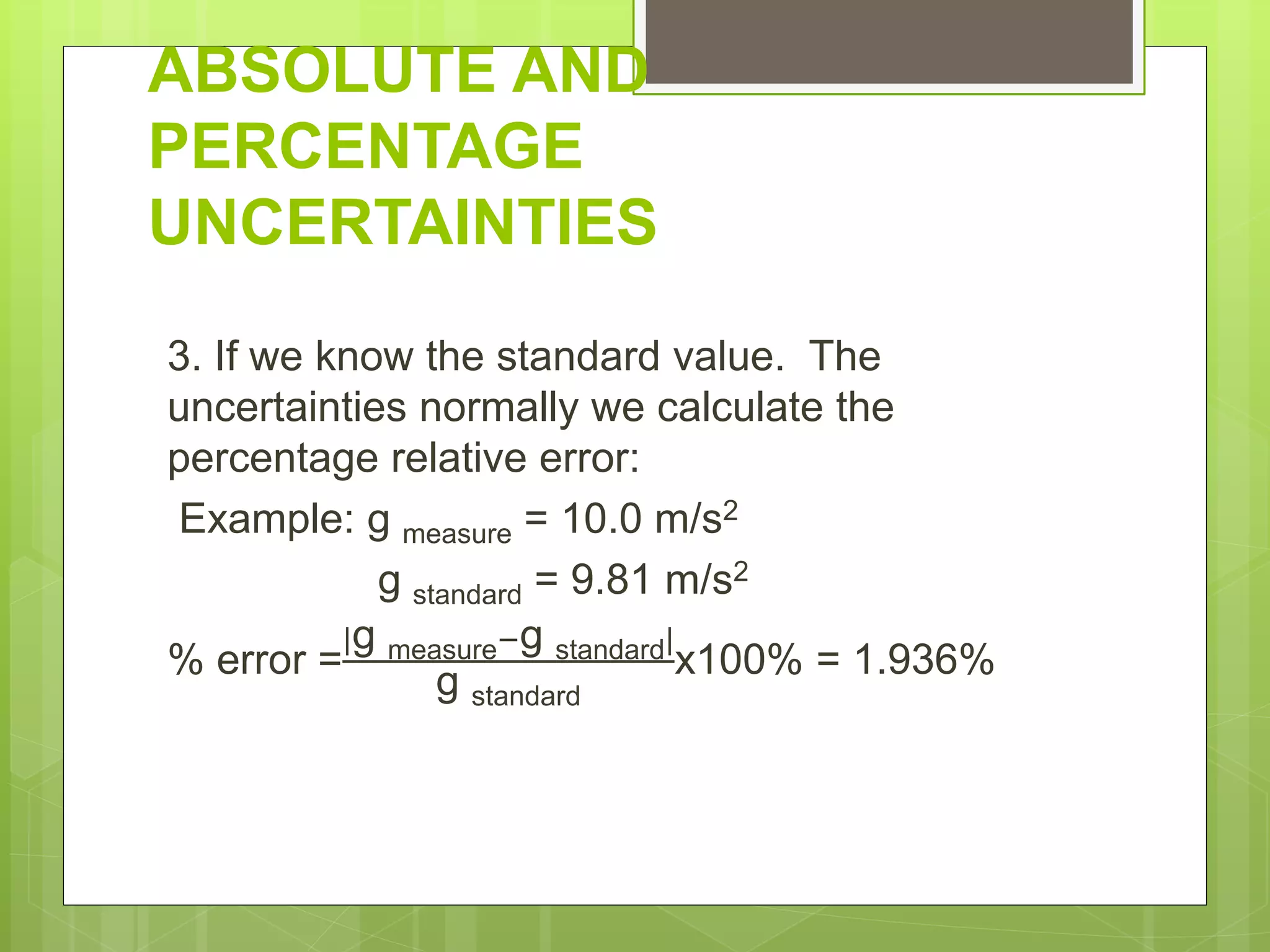
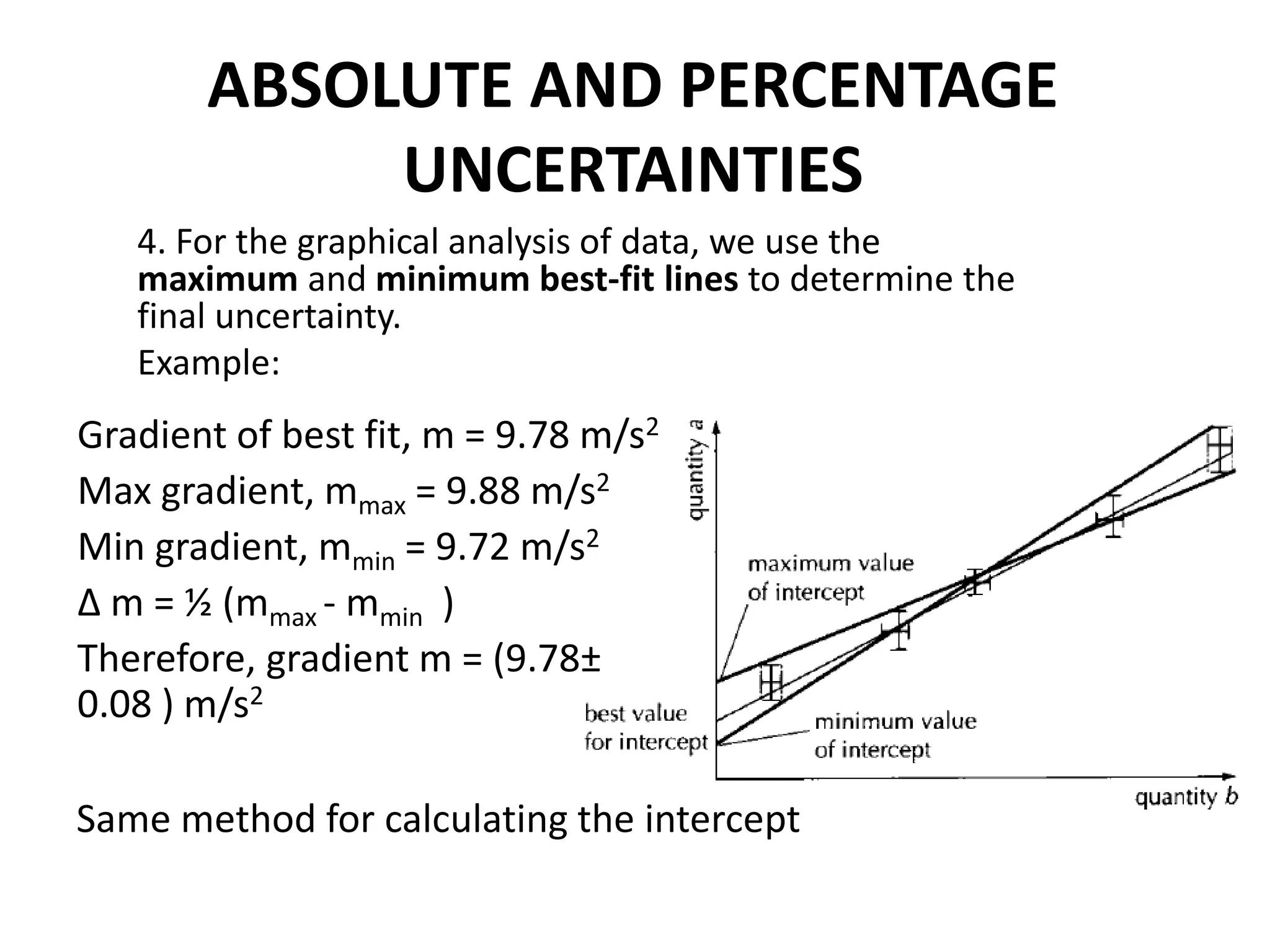
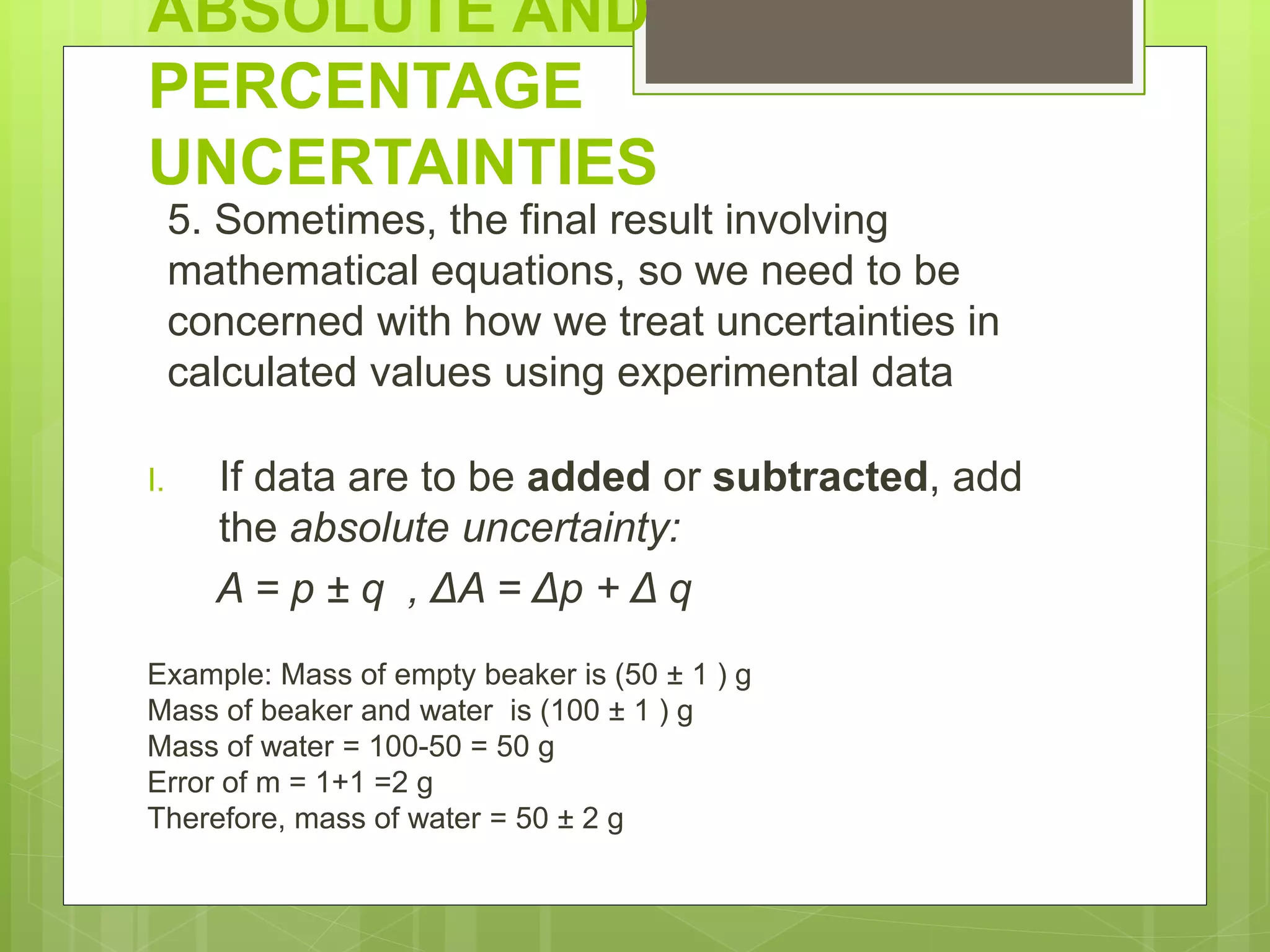
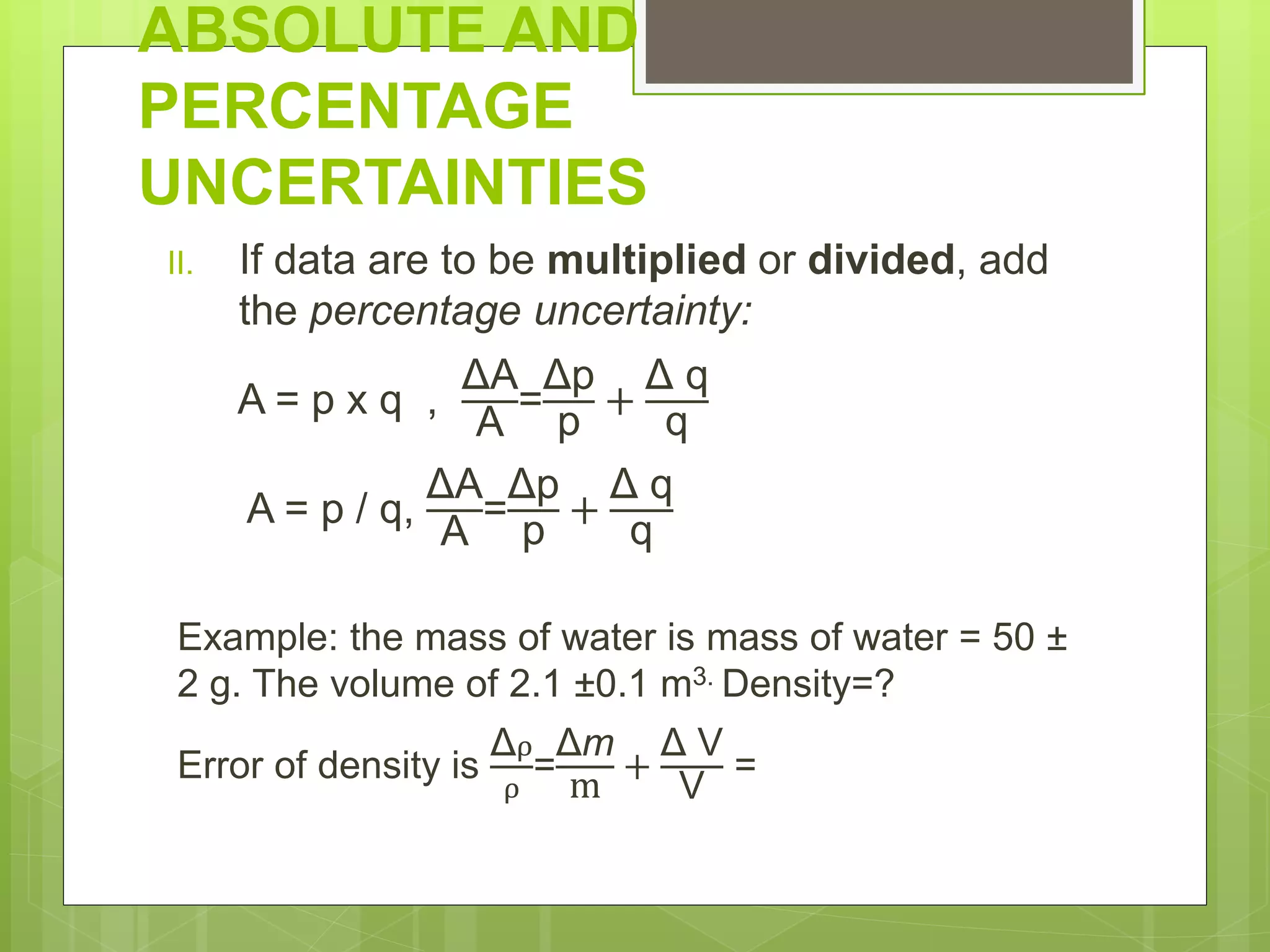
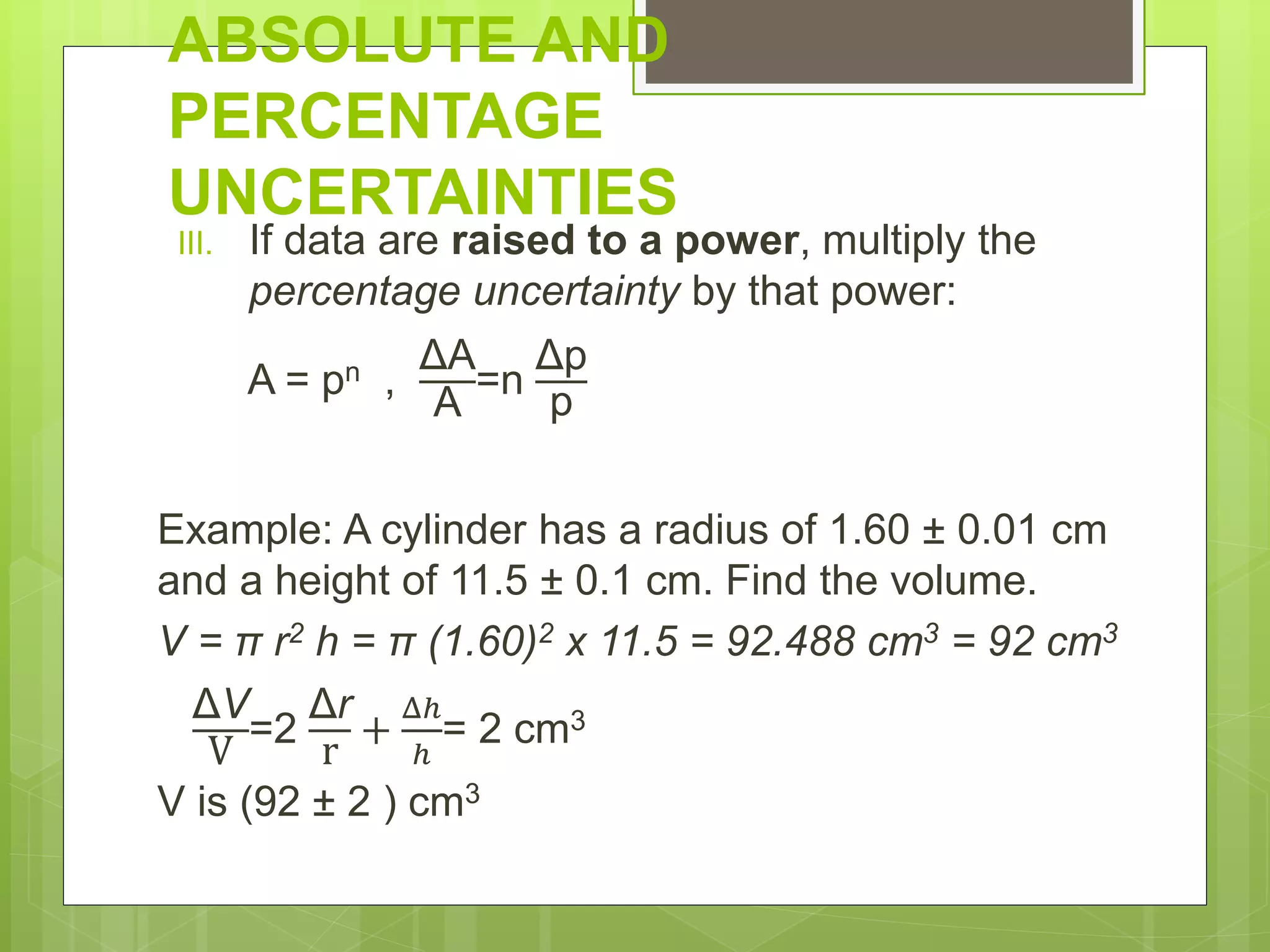
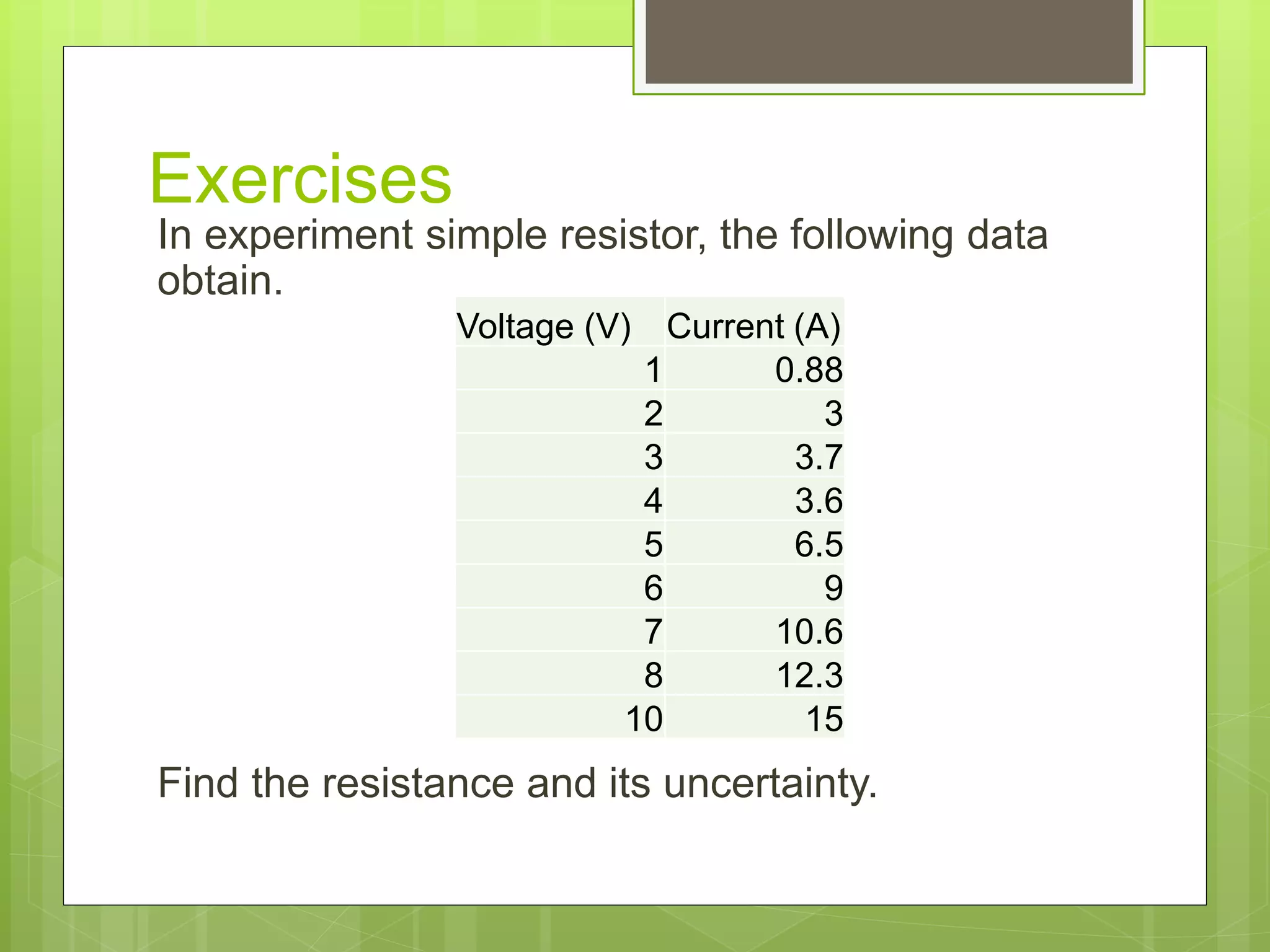
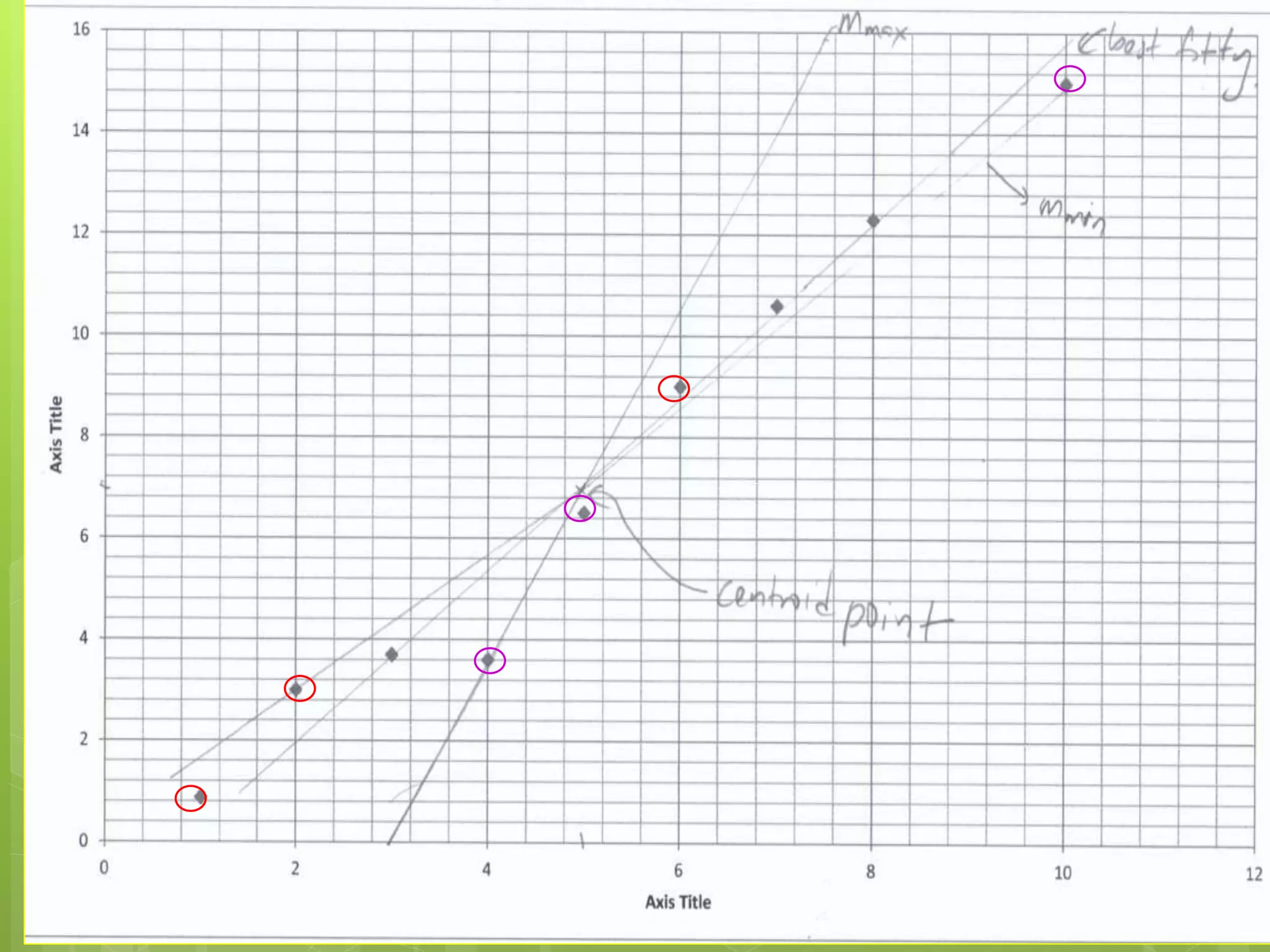
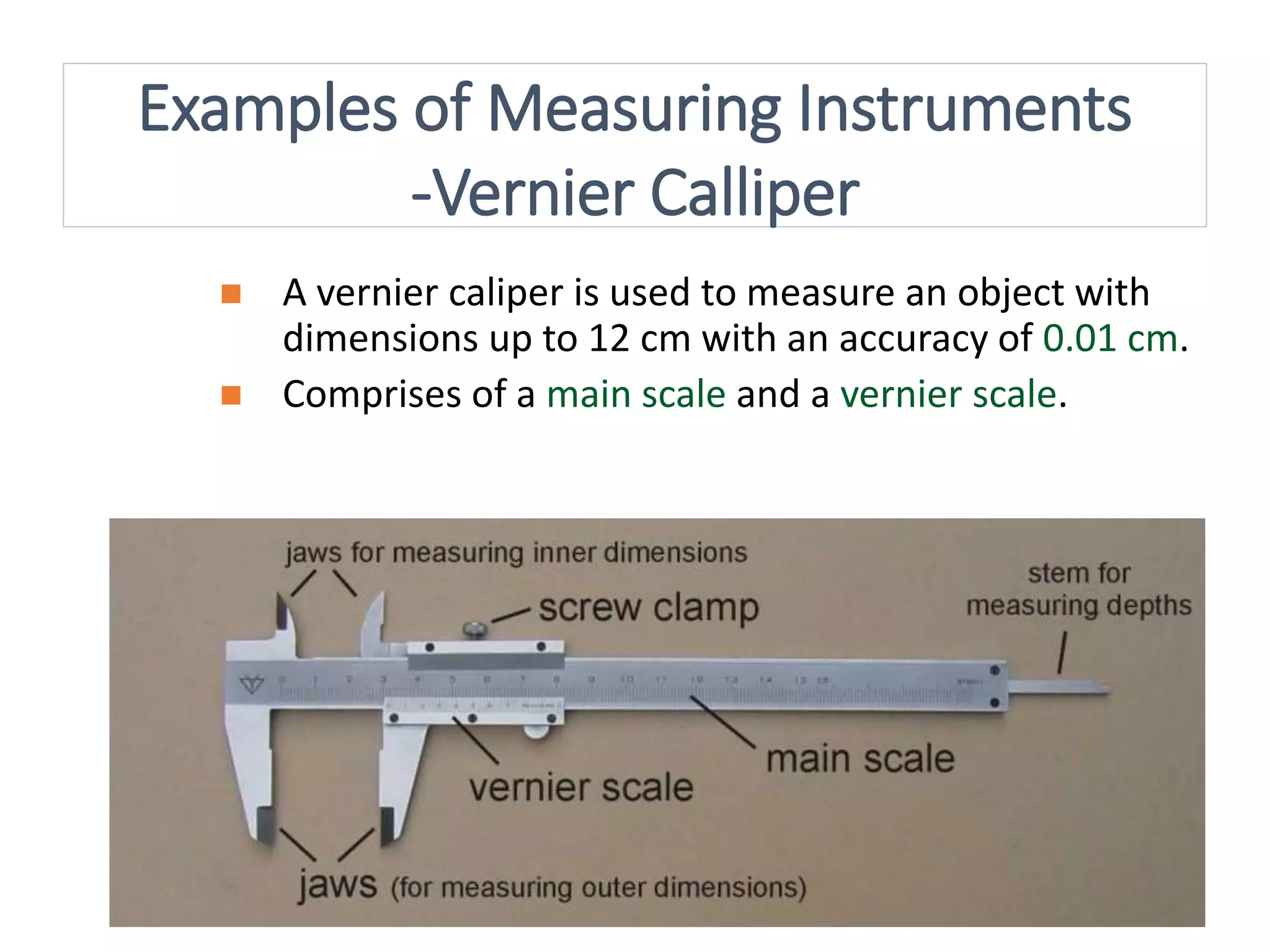
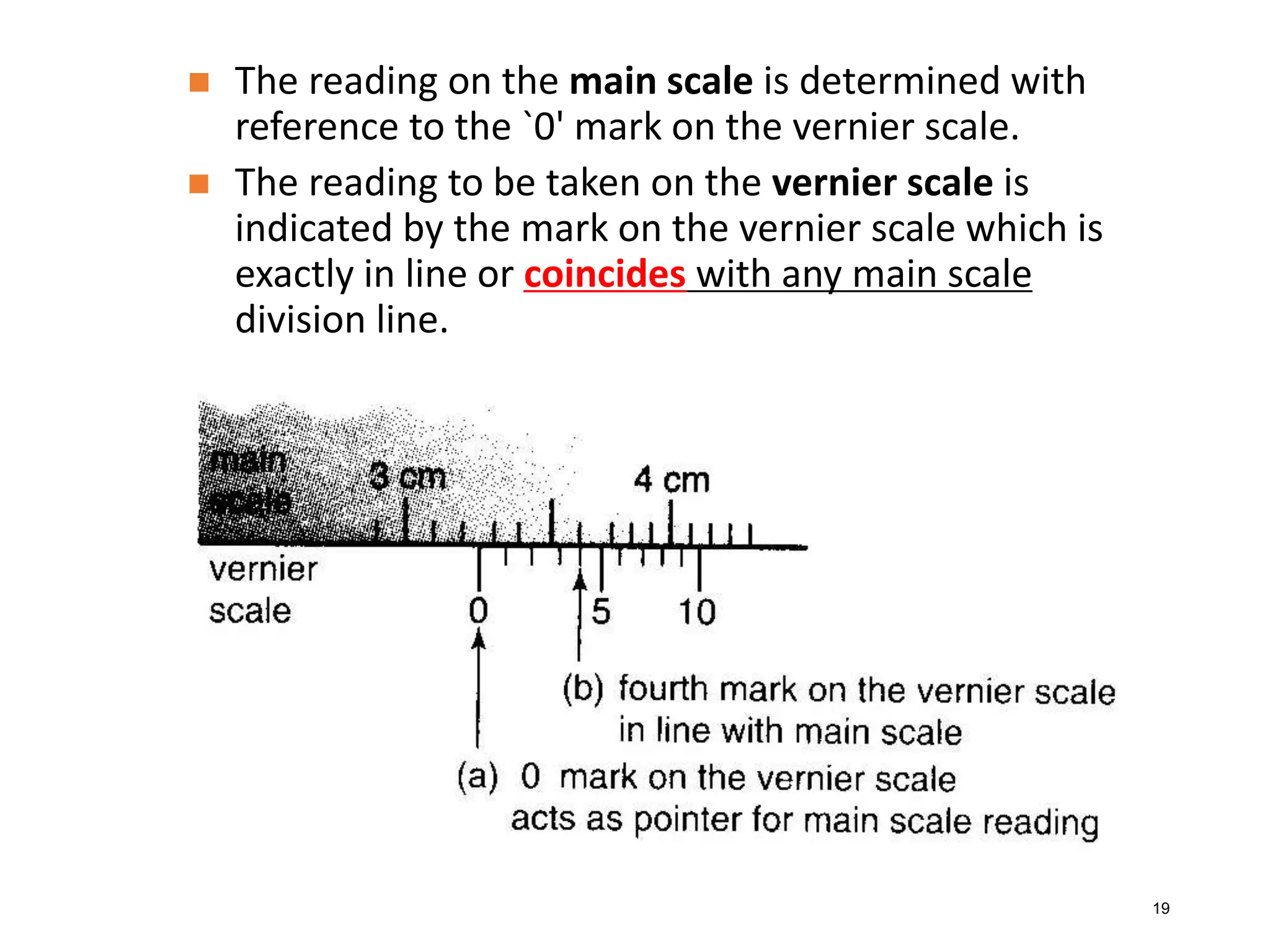
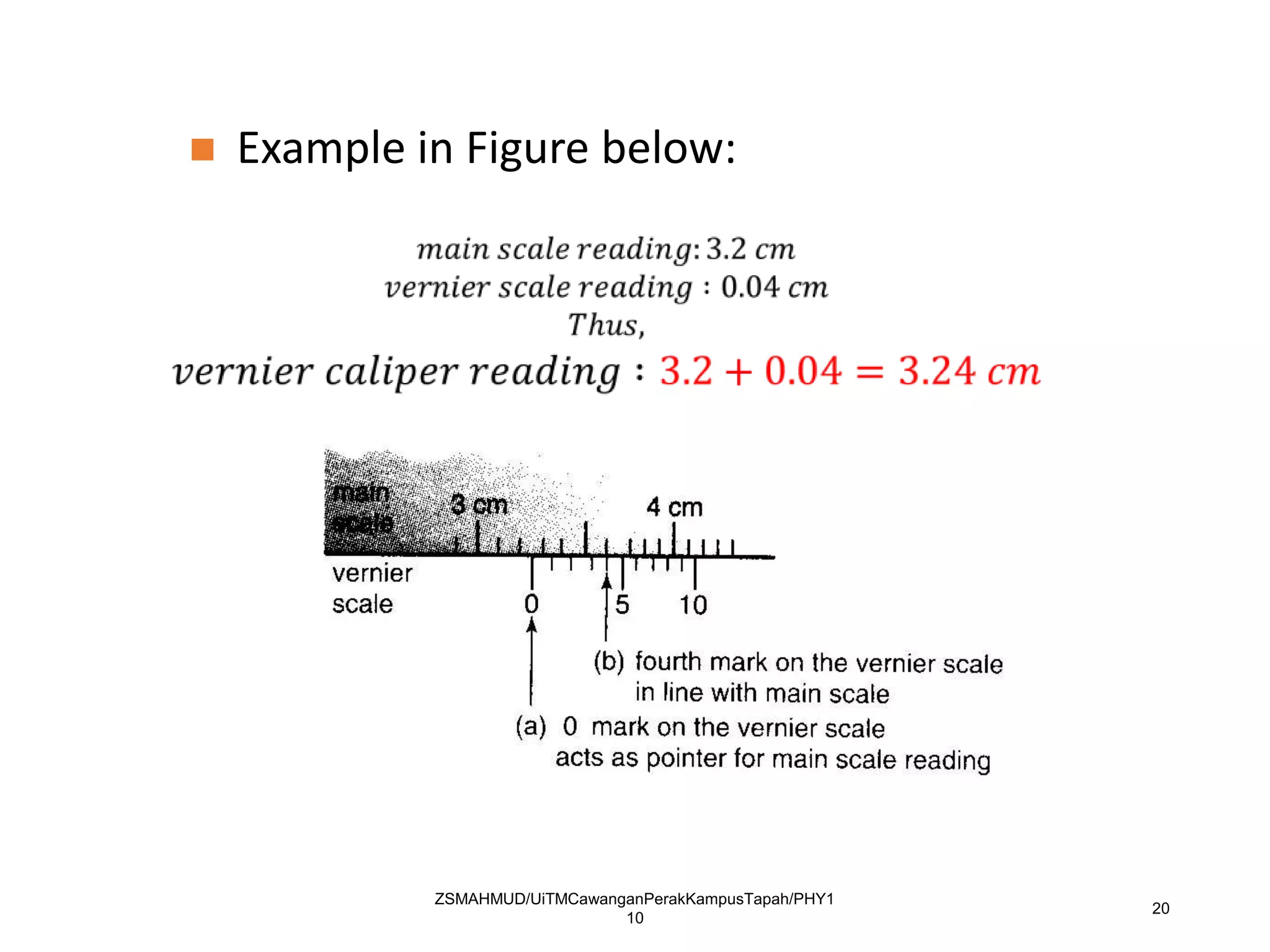
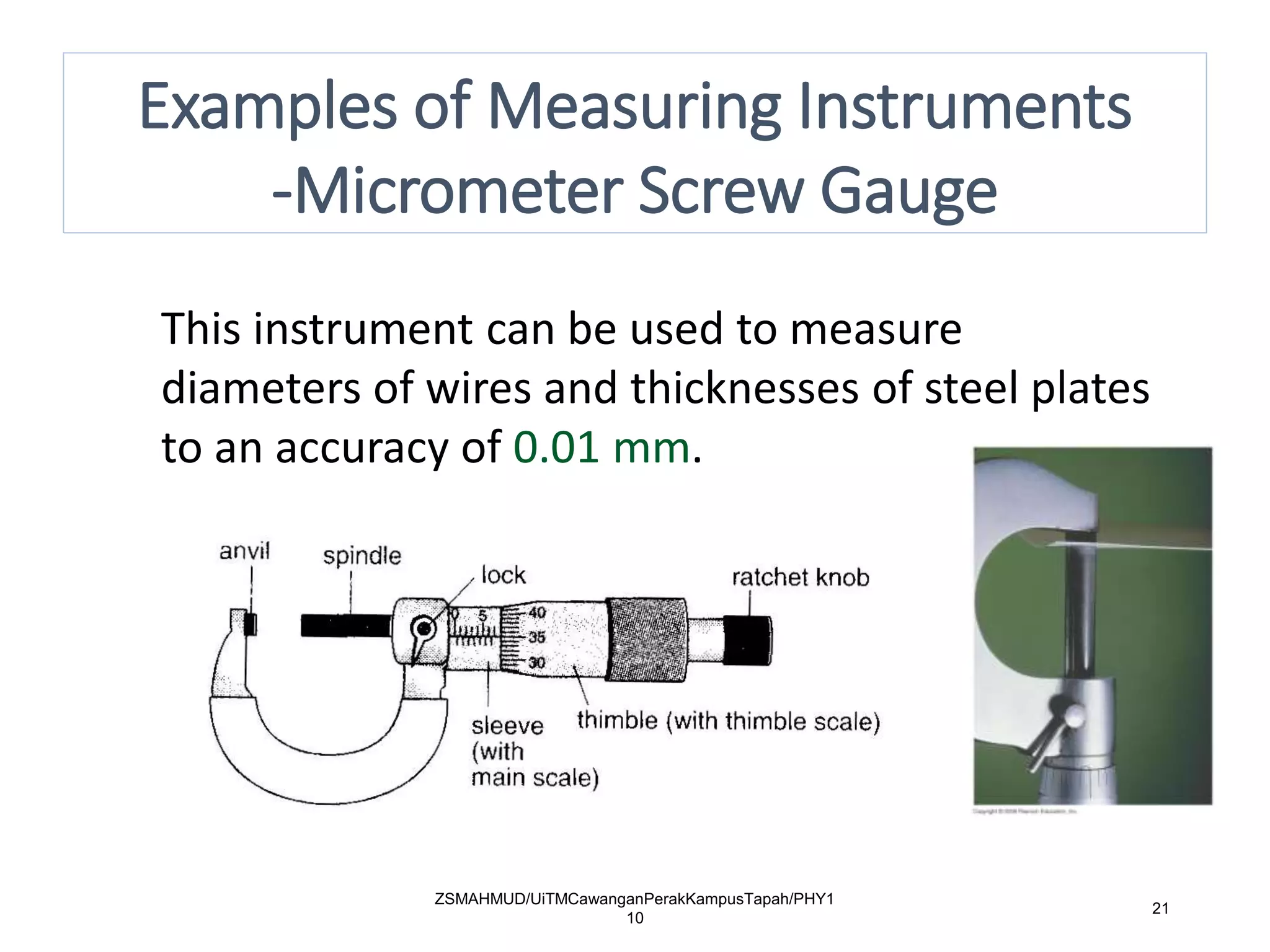
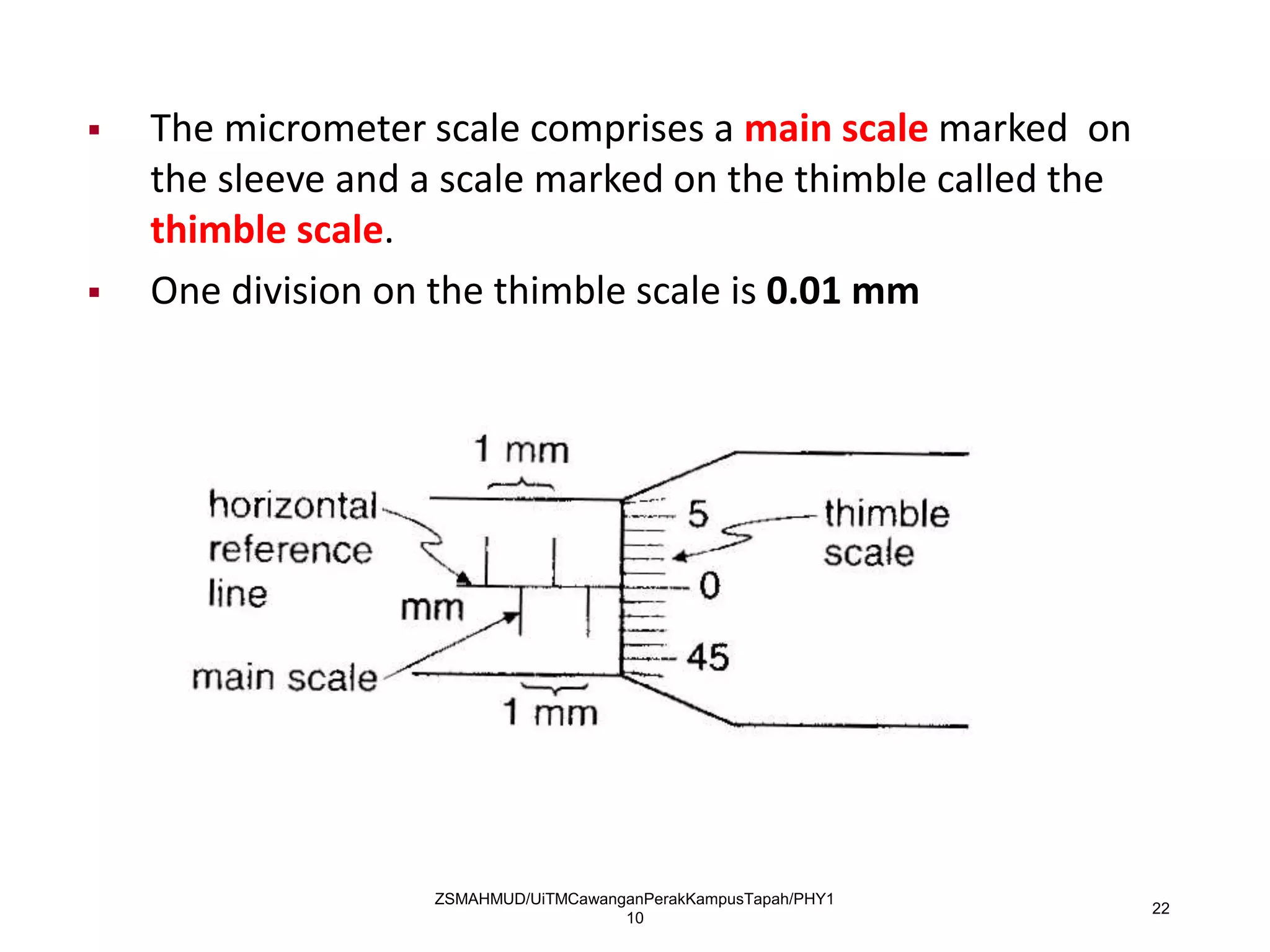
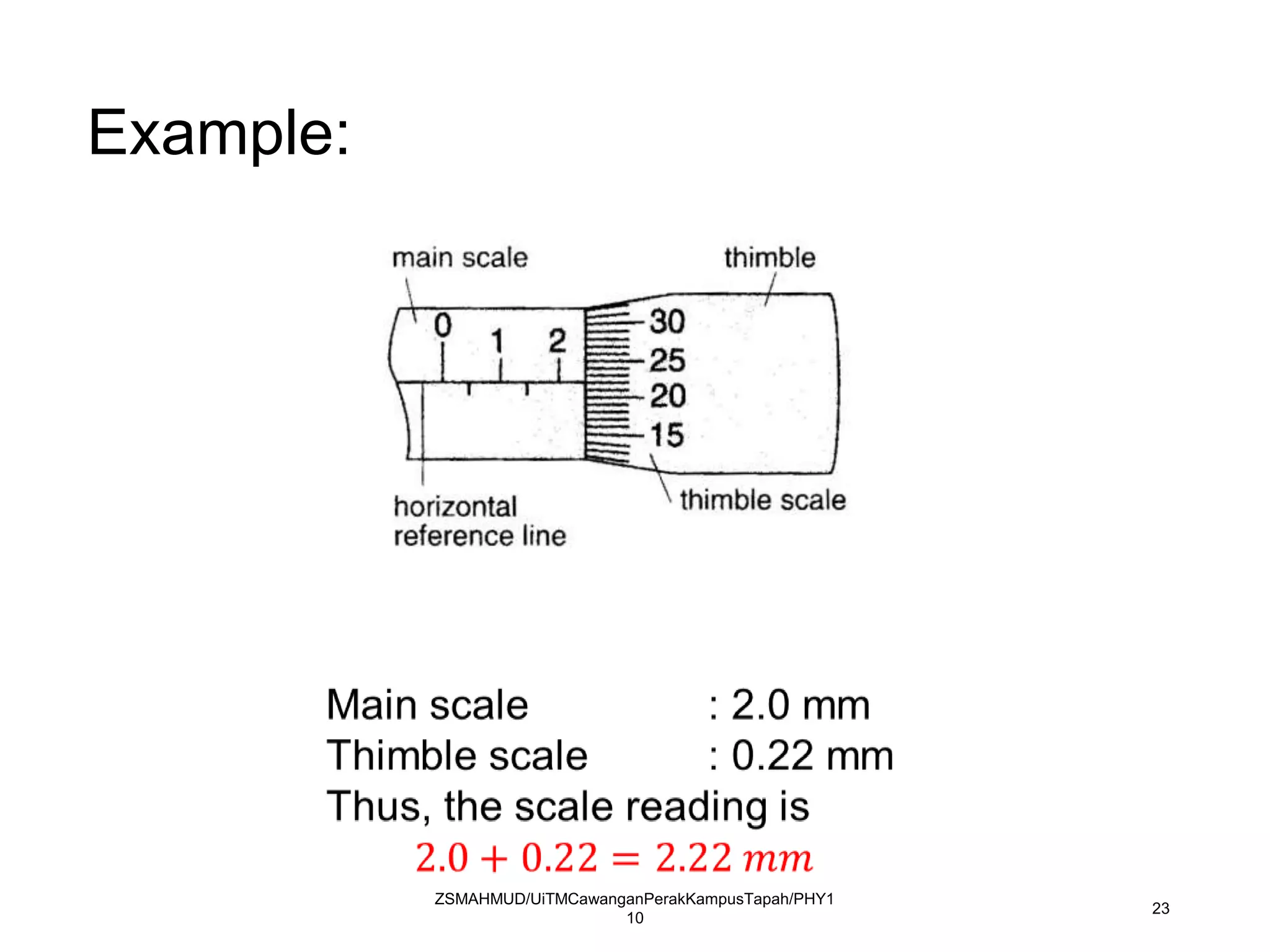
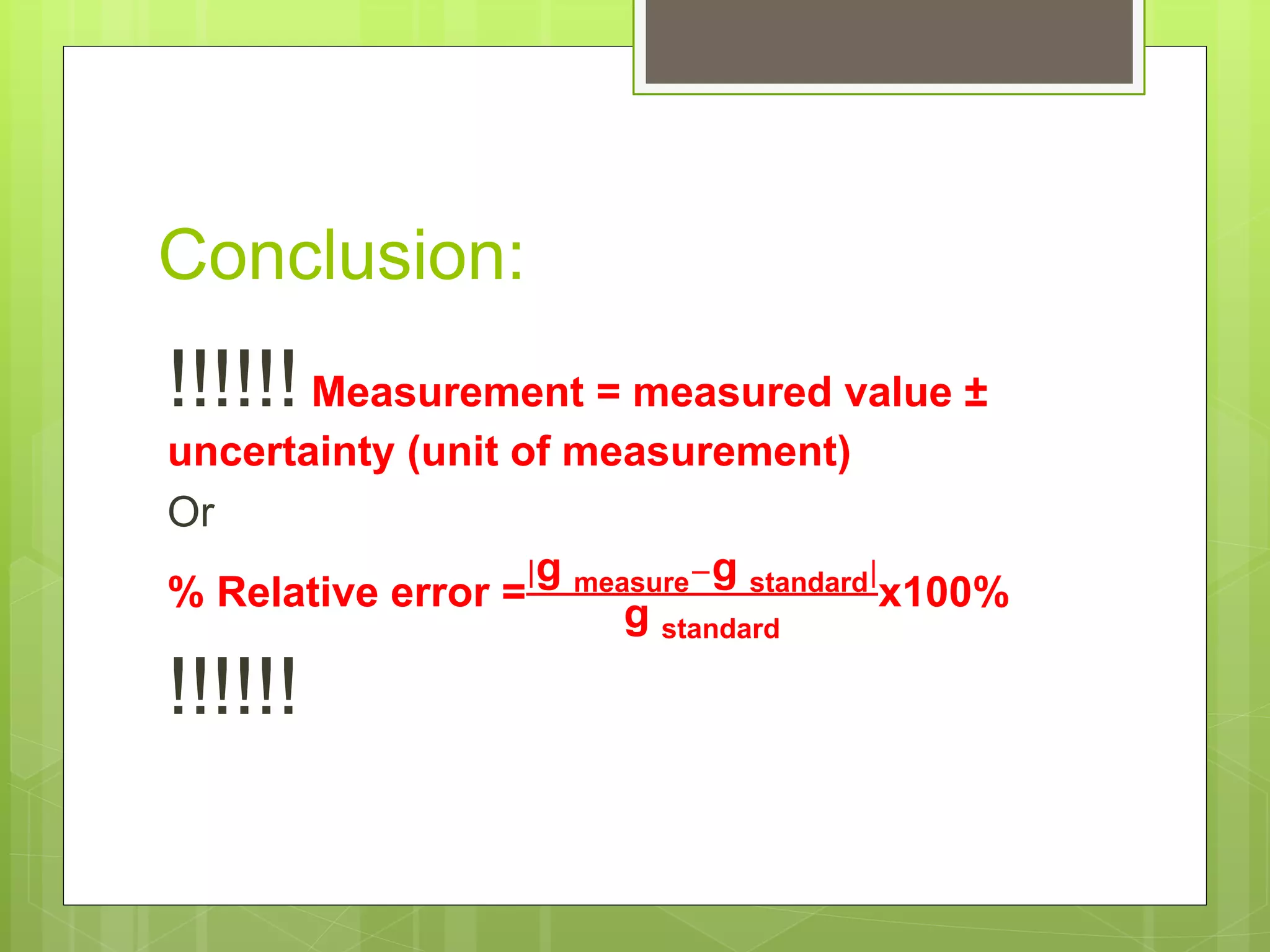
Measurement involves uncertainty from errors and imprecision. An error is the difference between measured and expected values, while uncertainty summarizes the error. Random errors arise from unpredictable fluctuations, and systematic errors are reproducible biases. Accuracy refers to closeness to the true value, while precision reflects consistency of measurements. Uncertainty is quantified from instrument resolution, repeated measurements, or comparison to a standard value. It is calculated and reported with the measurement result.