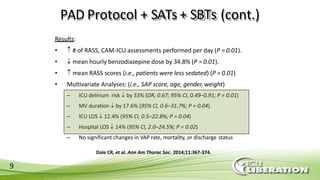
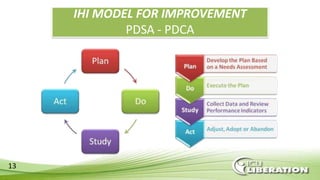
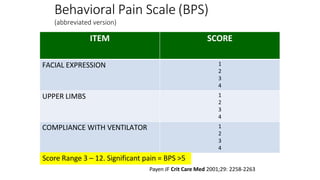
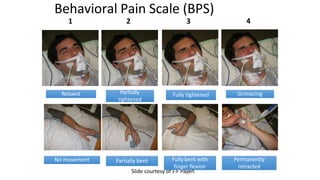
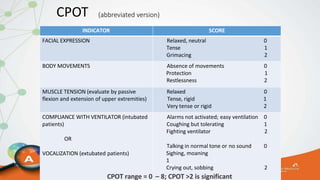
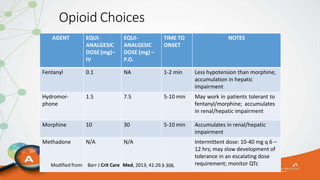
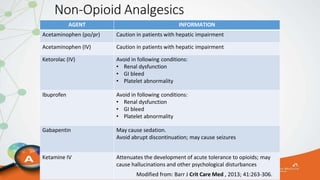
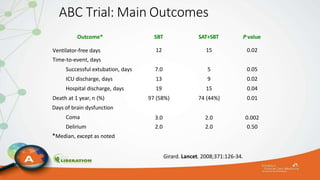
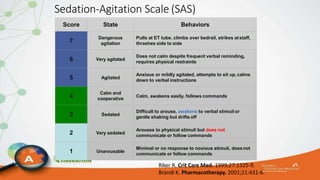
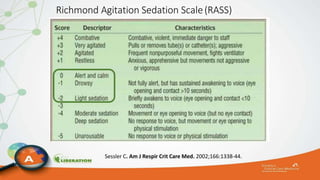
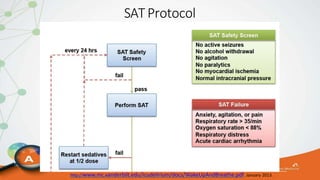
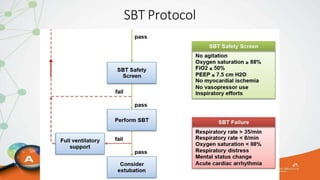
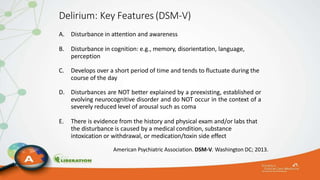
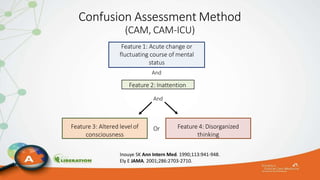
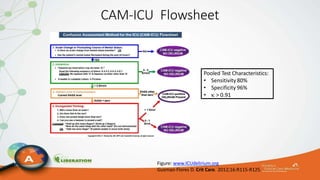
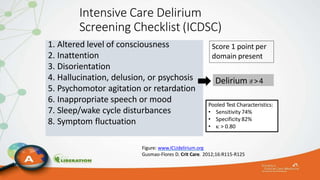
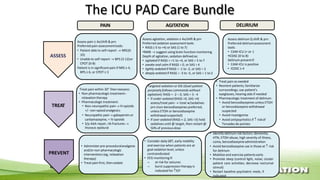
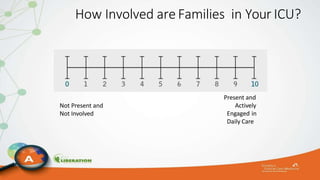
The document outlines the ABCDEF bundle, a set of guidelines aimed at improving ICU patient outcomes by focusing on pain management, sedation protocols, and early mobility. It highlights the importance of reducing deep sedation and ventilator dependence while preventing ICU delirium through coordinated care strategies. Results from various studies indicate that implementation of these guidelines can lead to reduced ICU length of stay, lower health care costs, and improved patient quality of life.