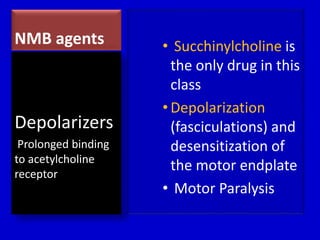
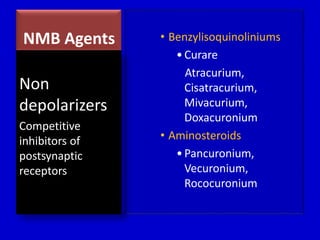
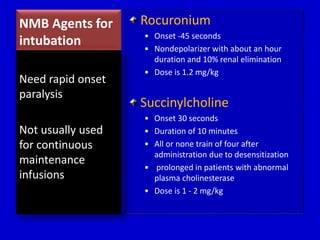
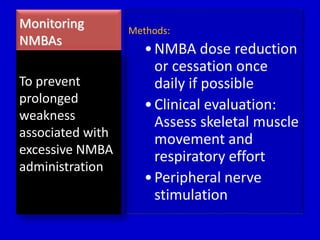
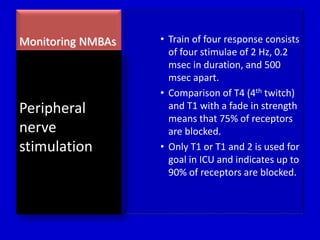
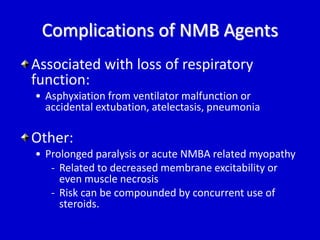
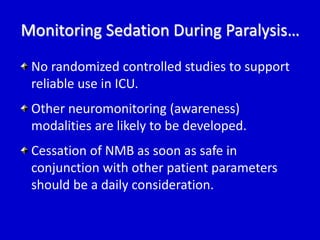
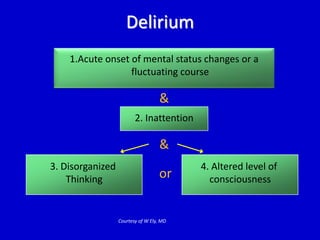
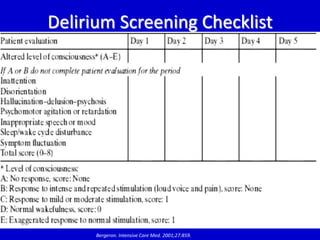
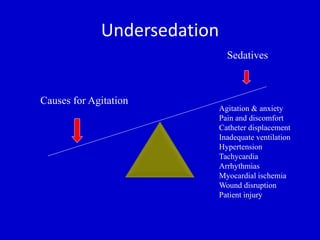
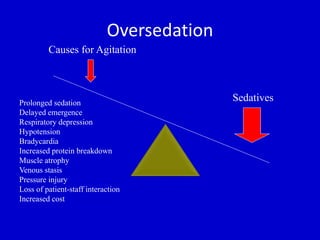
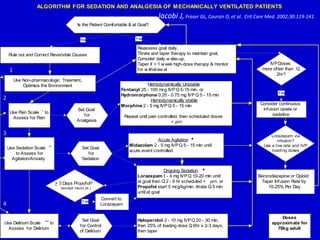
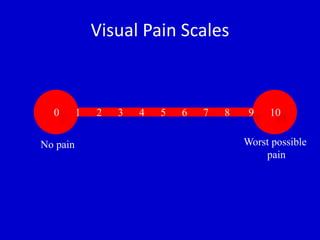
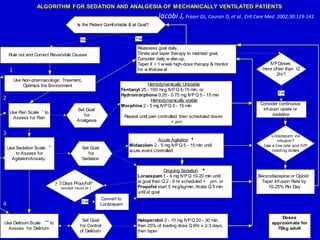
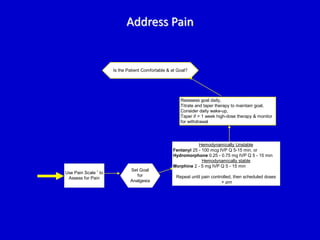
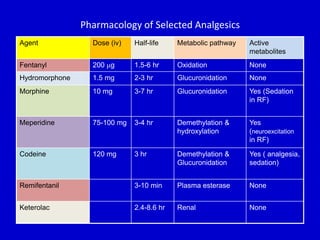
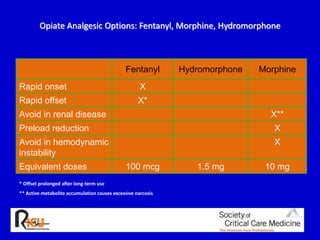
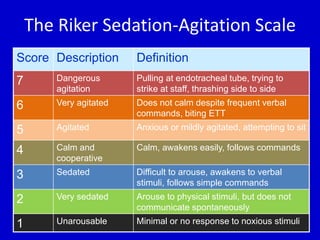
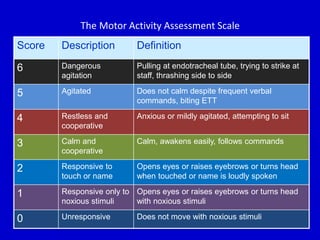
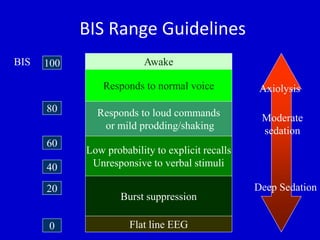
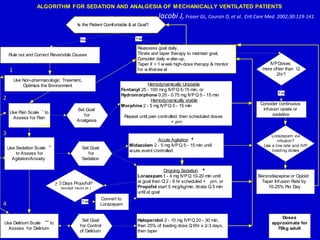
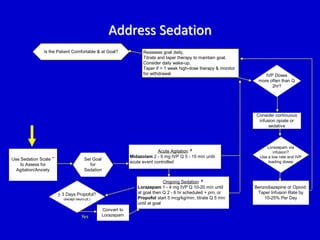
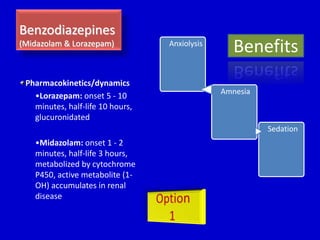
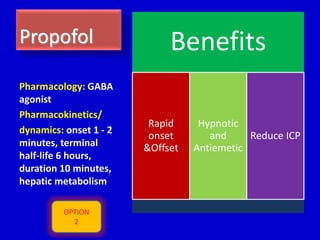
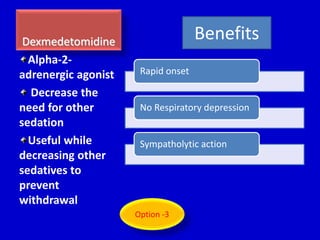
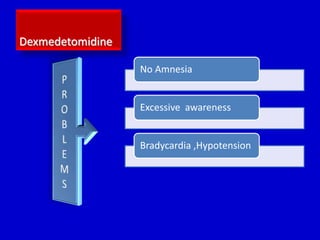
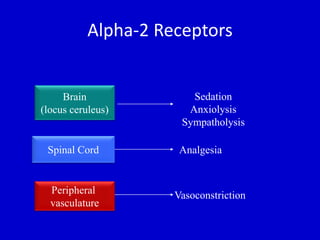
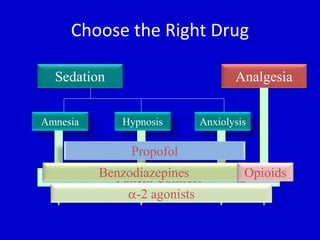
The document discusses guidelines for sedation, analgesia, and paralysis in the ICU. It notes that 50% of ICU patients experience agitation due to pain, delirium, anxiety, or sleep deprivation. Up to 70% of ICU patients have some recall of their time in the ICU, which can cause anxiety, fear, or PTSD. The optimal level of sedation allows for patient comfort while allowing interaction. Protocols for daily awakening and sedation titration can reduce length of stay, need for tracheostomies, and diagnostic evaluations. Validated scales like the Ramsay Sedation Scale and Sedation-Agitation Scale can help assess sedation levels, while the Numeric Rating Scale and Visual Analog Scale assess pain.













![Protocols and Assessment Tools
Sedation
o Validated sedation
assessment tools (Ramsay
Sedation Scale [RSS],
o Sedation-Agitation Scale
[SAS]
o Richmond Sedation-agitation
Scale [RSAS]
No evidence that one is preferred over
another
Pain
o Numeric rating scale
[NRS]
o Visual analogue scale
[VAS]
Pain assessment tools - none validated
in ICU](https://image.slidesharecdn.com/sedationanalgesiaparalysis-141020020713-conversion-gate01/85/Sedation-analgesia-paralysis-17-320.jpg)
























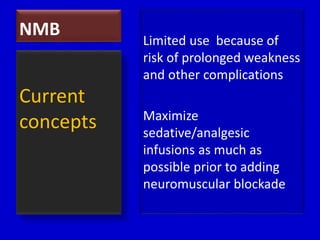
![NMB • Facilitate mechanical
ventilation [abdominal compartment
syndrome, high airway pressures, and
dyssynchrony]
• High Frequency Ventilation,
Prone ventilation
• Elevated intracranial
pressures
• Reduce oxygen consumption
• Prevent muscle spasm
[neuroleptic malignant syndrome, tetanus, etc.]
• Protect surgical wounds or
medical device placement
When to Use it?](https://image.slidesharecdn.com/sedationanalgesiaparalysis-141020020713-conversion-gate01/85/Sedation-analgesia-paralysis-64-320.jpg)