This document provides information about arterial blood gas (ABG) analysis, including how to interpret levels of oxygenation, ventilation, and acid-base imbalance from an ABG test. It discusses key measurements like PaO2, PaCO2, oxygen saturation, and bicarbonate levels. It also reviews respiratory physiology concepts like ventilation-perfusion mismatch, shunt fraction, and the oxygen-hemoglobin dissociation curve that are important for understanding ABG results.






![V/Q Mismatch is the commonest cause of hypoxia*
V/Q: Ideal -1, Real life: 0.3-2.1
What will be the V/Q in these alveoli?
This dead space adds to apparatus dead space and can
pCO2 [dead space causes less hypoxia than shunt]
Facebook page: Anesthesia Info from The
Normal ratio of Dead space
ventilation to Vt is 0.3; When >0.5
CO2 will increase](https://image.slidesharecdn.com/arterialbloodgasanalysisfinal-230326121310-d3efb412/85/ARTERIAL-BLOOD-GAS-ANALYSIS-FINAL-pptx-7-320.jpg)
![The concept of ‘Mixed Venous Sample’
Venous effluents from different organs have different oxygen
content
How a single sample can represent the whole body?
Pulmonary Artery Catheter (PAC)
Mixed Venous PO2 [PvO2] 40 mm of Hg
Mixed Venous saturation [SvO2] 75%
In low CO states with continuing O2 extraction, PvO2 will be
low
Sample from a CVC [if no PAC] can serve as an alternative
Facebook page: Anesthesia Info from The](https://image.slidesharecdn.com/arterialbloodgasanalysisfinal-230326121310-d3efb412/85/ARTERIAL-BLOOD-GAS-ANALYSIS-FINAL-pptx-10-320.jpg)

![Travel [of O2] and Living [of humans]
When air is inhaled, it gets saturated with water
vapour. So to find out the alveolar partial
pressure of O2, water vapour pressure has to
be substracted..also the mean airway pressure!](https://image.slidesharecdn.com/arterialbloodgasanalysisfinal-230326121310-d3efb412/85/ARTERIAL-BLOOD-GAS-ANALYSIS-FINAL-pptx-13-320.jpg)
![Travel [of O2] and Living [of humans]
Alveolar partial pressure of O2
713 x FiO2 – 1.25 x PaCO2
PAO2 =[(PB – PH2O) FiO2 ] – (PaCO2 / RQ)
Atmospheric pressure is 760 mm Hg at sea level
PH2O is vapor pressure of water at 37°C and is equal to 47 mmHg
The respiratory quotient or respiratory coefficient (RQ) is the ratio of CO2
produced divided by the O2 consumed, and its value is typically 0.8 (RQ
= CO2 eliminated / O2 consumed). R is taken as ! @FiO2> 0.6
PB – PH2O is known as PiO2 713
Simplified as
Facebook page: Anesthesia Info from The
PAO2 = 713 x FiO2 – 1.25 x
PaCO2](https://image.slidesharecdn.com/arterialbloodgasanalysisfinal-230326121310-d3efb412/85/ARTERIAL-BLOOD-GAS-ANALYSIS-FINAL-pptx-14-320.jpg)

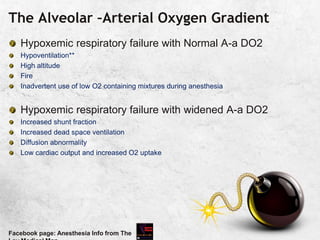
![The Alveolar –Arterial Oxygen Gradient
As O2 reaches blood by diffusion, the expected PaO2 will be
less than PAO2 [ suspect air bubble, in ABG sample, if > 100 in patient
breathing room air]
Known as Alveolar –Arterial Oxygen Gradient
10-15 mm in young to middle aged
PaO2= 109- 0.43 [age in years]
It increases with increase in FiO2 [@FiO2 of 1,110!)
If higher than expected for age, shunt fraction is high
Patient should receive 100% O2 for 15 minsIdeally PaO2 should be 550; every 20 mm
Hg difference is equal to 1% shunt
Facebook page: Anesthesia Info from The](https://image.slidesharecdn.com/arterialbloodgasanalysisfinal-230326121310-d3efb412/85/ARTERIAL-BLOOD-GAS-ANALYSIS-FINAL-pptx-16-320.jpg)

![Real life situation*
Patient breathing room air, has PaO2 90 mm of Hg, SpO2
96%, and PaCO2 110 mm of Hg
Apply Alveolar Gas Equation
[713x0.2]-[1.2x110]= PAO2 is 18!, but SpO2 is 96. So one
among the value is wrong.
Facebook page: Anesthesia Info from The](https://image.slidesharecdn.com/arterialbloodgasanalysisfinal-230326121310-d3efb412/85/ARTERIAL-BLOOD-GAS-ANALYSIS-FINAL-pptx-19-320.jpg)
![CaO2- Oxygen Content
Oxygen carried as oxyhemoglobin + dissolved O2
CaO2= [1.39 X Hb (gm/dl) X Saturation] + 0.003 X PaO2
1.39 is the amount of O2 in ml, that will bind to 1 gm of Hb
0.003 is the solubility coefficient of O2
If Hd=15 g/dl, SaO2 99%, 20.4 ml as oxy Hb + 0.3 ml in
plasma20.7
Facebook page: Anesthesia Info from The](https://image.slidesharecdn.com/arterialbloodgasanalysisfinal-230326121310-d3efb412/85/ARTERIAL-BLOOD-GAS-ANALYSIS-FINAL-pptx-20-320.jpg)
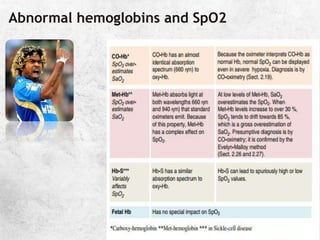
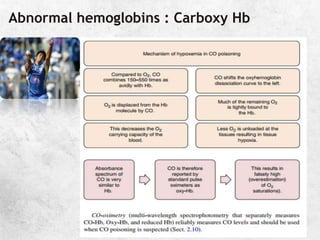

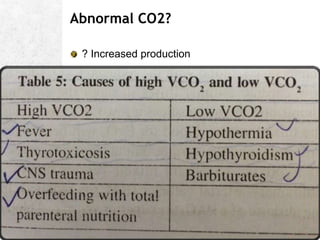
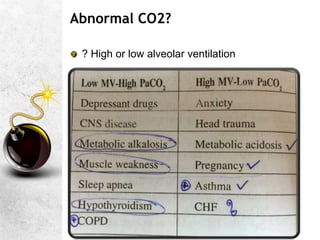
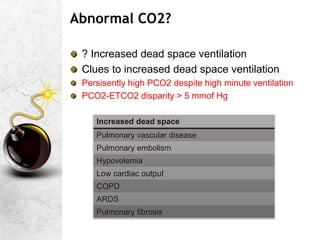

![A great quote!
O
pKa is the negative logarithm of the dissociation constant
pKa' s value is dependent on the temperature,[H+] and the
ionic concentration of the solution. It has a value of 6.1 @
37C and pH of 7.4
Facebook page: Anesthesia Info from The](https://image.slidesharecdn.com/arterialbloodgasanalysisfinal-230326121310-d3efb412/85/ARTERIAL-BLOOD-GAS-ANALYSIS-FINAL-pptx-28-320.jpg)
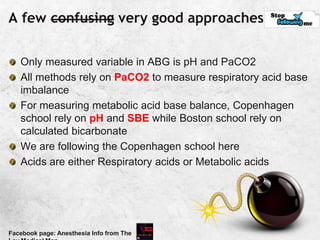
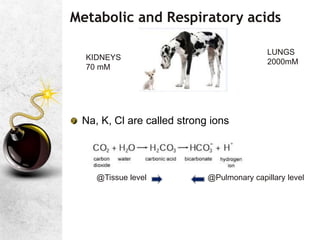
![Unity in Diversity; concept of SBE
HCO3 may be the primary mover in metabolic imbalance
But there are other Non carbonic buffer systems
The concept of SBE makes this multi-buffer system into a
hypothetical system, where the entire body behaves like a
bicarbonate solution
BE[actual] = BE of whole blood
SBE = BE of ECF
Facebook page: Anesthesia Info from The](https://image.slidesharecdn.com/arterialbloodgasanalysisfinal-230326121310-d3efb412/85/ARTERIAL-BLOOD-GAS-ANALYSIS-FINAL-pptx-31-320.jpg)
![This is how I did it! Hmm tasty
Nullify respiratory effects: make PaCO2: 40 and Temp 370 C
Assume pH of sample as alkaline titrate HCl till pH becomes
7.4 the amount of HCl required is the amount of excess
acids[=BE]. [Similarly excess acids titrated by NaOH -BE or
Base deficit
Titratable hydrogen ion concentration is a better term than Base
excess or Deficit. Ok.. One question from ECF “ Oh. Why you
are neglecting me??? You want that silly blood alone?? “
Facebook page: Anesthesia Info from The
ECF is the fluid through which acid base changes are mediated.
Create a hypothetical ECF compartment by diluting the arterial
Blood 3 fold by its own plasma. Now report BE of this compartment:
Your delicious dish is ready: SBE is the best measure of
assessing metabolic acid base changes; Reported in
mM/L Normal: ± 2 mM/L](https://image.slidesharecdn.com/arterialbloodgasanalysisfinal-230326121310-d3efb412/85/ARTERIAL-BLOOD-GAS-ANALYSIS-FINAL-pptx-32-320.jpg)

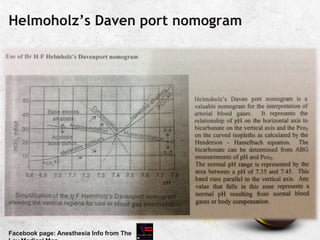
![pH and [H+]
](https://image.slidesharecdn.com/arterialbloodgasanalysisfinal-230326121310-d3efb412/85/ARTERIAL-BLOOD-GAS-ANALYSIS-FINAL-pptx-35-320.jpg)




![Salute our buffers!
Bicarbonate buffer system is the major buffer system in the
ECF.
Responsible for about 80% of extracellular buffering.
It can't buffer respiratory acid base disorders [Bicarbonate
system cannot buffer changes in H+ produced by the
reaction between CO2 and H2O]
Facebook page: Anesthesia Info from The](https://image.slidesharecdn.com/arterialbloodgasanalysisfinal-230326121310-d3efb412/85/ARTERIAL-BLOOD-GAS-ANALYSIS-FINAL-pptx-40-320.jpg)


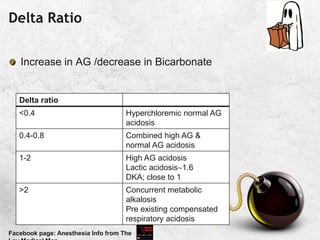
![ANION GAP
When all the commonly measured anions are substracted
from the cations, the result is a positive value of 12±4 mEq/L
Due to unmeasured anions
Corrected AG = Calculated AG + 2.5 [4.5-measured albumin
in g/dl]
Facebook page: Anesthesia Info from The](https://image.slidesharecdn.com/arterialbloodgasanalysisfinal-230326121310-d3efb412/85/ARTERIAL-BLOOD-GAS-ANALYSIS-FINAL-pptx-43-320.jpg)





![ACUTE RESPIRATORY ACID BASE CHANGES
PaCO2 pH SBE=0
• ACUTE RESPIRATORY ACIDOSIS[
buffering only; 99% in ICF]
PaCO2 pH
• ACUTE RESPIRATORY ALKALOSIS
Facebook page: Anesthesia Info from The](https://image.slidesharecdn.com/arterialbloodgasanalysisfinal-230326121310-d3efb412/85/ARTERIAL-BLOOD-GAS-ANALYSIS-FINAL-pptx-54-320.jpg)








![Metabolic Acidosis : effects
Decreased strength of respiratory muscles
Hyperventilation
Myocardial depression
Sympathetic over activity
Decreased arrhythmia threshold
Resistance to catecholamines
Hyperkalemia
Increased metabolic demand [N:5%of VO2; in distress 25%]
Insulin resistance
Facebook page: Anesthesia Info from The](https://image.slidesharecdn.com/arterialbloodgasanalysisfinal-230326121310-d3efb412/85/ARTERIAL-BLOOD-GAS-ANALYSIS-FINAL-pptx-67-320.jpg)
![Urinary Anion Gap [UAG]
=UA-UC=[Na]+[K]-[Cl]
If acidosis is due to loss of base via bowel, kidneys will try to
increase [H+] losswith NH4+ & Cl- in urineUAG
So in a patient with hyperchloremic metabolic acidosis:
Negative UAG GIT loss of [HCO3-]
Positive UAG Loss of base via kidney (problem is with
kidney and it cant increase ammonium excretion)
“neGUTive”
Facebook page: Anesthesia Info from The](https://image.slidesharecdn.com/arterialbloodgasanalysisfinal-230326121310-d3efb412/85/ARTERIAL-BLOOD-GAS-ANALYSIS-FINAL-pptx-68-320.jpg)
![Metabolic Alkalosis
Generally pCO2 wont go > 55; if > 55, indicates severe
alkalosis OR combined metabolic alkalosis + respiratory
acidosis
Usually [HCO3-] prompt [HCO3-] excretion by kidney;
persistence requires additional process to impair [HCO3-]
excretion](https://image.slidesharecdn.com/arterialbloodgasanalysisfinal-230326121310-d3efb412/85/ARTERIAL-BLOOD-GAS-ANALYSIS-FINAL-pptx-71-320.jpg)

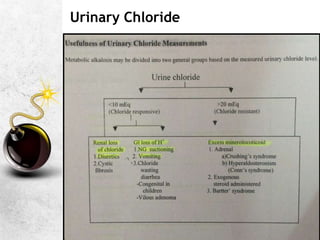
![Additional points- Metabolic alkalosis
Depresses respiration hypoxemia & hypercarbia
Effects on PaCO2 are seen only when HCO3> 35 Mm/L
Chloride responsive [Urinary Cl- < 15 mEq/L]: Rx is chloride-
volume-potassium repletion [ If severe infusion of 0.1N HCl
Chloride resistant [Urinary Cl- >25 mEq/L]: Rx is correction of
the cause of mineralocorticoid excess and potassium
depletion
Selective HCO3 excreting diuretic Acetazolamide
Facebook page: Anesthesia Info from The](https://image.slidesharecdn.com/arterialbloodgasanalysisfinal-230326121310-d3efb412/85/ARTERIAL-BLOOD-GAS-ANALYSIS-FINAL-pptx-73-320.jpg)
![Curious facts
Hepatic metabolism of citrate, lactate, acetate--. Brief
alkalosis
Chloride and bicarbonate are the only anions present in
appreciable amounts in ECF: so a defiency in one must lead
to an increase in the other to maintain electroneutrality
Vomiting and diuretics cause chloride depletion
Mineralocorticoid excess [in Cushings, the excess
corticosteroids have some mineralocorticoid effects]K+ &
H+ loss matched by [HCO3-]
Facebook page: Anesthesia Info from The](https://image.slidesharecdn.com/arterialbloodgasanalysisfinal-230326121310-d3efb412/85/ARTERIAL-BLOOD-GAS-ANALYSIS-FINAL-pptx-74-320.jpg)





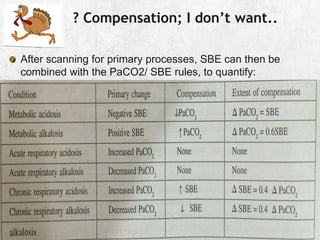



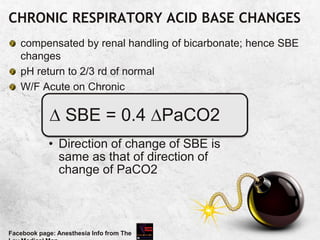
![Example:5
.
?alkalosis, ?respiratory, expected= 0.4x 16[40-24]=6
Compensated respiratory alkalosis
Facebook page: Anesthesia Info from The
pH:
PaCO2:
HCO3:
BE:
7.44
24
16
-6](https://image.slidesharecdn.com/arterialbloodgasanalysisfinal-230326121310-d3efb412/85/ARTERIAL-BLOOD-GAS-ANALYSIS-FINAL-pptx-90-320.jpg)
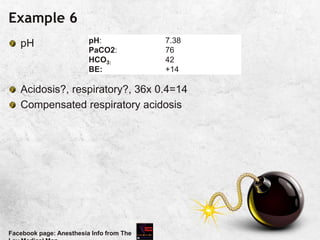
![Example 8
Alkalosis, respiratory?, ?metabolic compensation 0.4x 14=
5.6 [ direction: same as CO2]
Facebook page: Anesthesia Info from The
pH:
PaCO2:
HCO3:
BE:
7.44
26
18
-4](https://image.slidesharecdn.com/arterialbloodgasanalysisfinal-230326121310-d3efb412/85/ARTERIAL-BLOOD-GAS-ANALYSIS-FINAL-pptx-93-320.jpg)







